Module 2: Climatology of Water
Module 2: Climatology of Water
Overview
In this module, we will investigate the underlying causes of variations in precipitation on Earth, with a specific focus on large-scale climate belts and the role of mountain ranges in affecting the distribution of rainfall (and snow). The goals of the module are to develop a quantitative understanding of the physical processes that control the distribution of precipitation, and which ultimately govern regions where water is abundant and where it is scarce, both across the U.S. and globally. As part of this, you’ll develop facility with the concepts of relative humidity, saturation, water vapor content in the air, and how these vary with changes in temperature - all of which play a key role in determining when and where precipitation falls.
Goals and Learning Objectives
Goals and Learning Objectives
Goals
- Explain the distribution and dynamics of water at the surface and in the subsurface of the Earth
- Interpret graphical representations of scientific data
Learning Objectives
In completing this module, you will:
- Identify the unique physical properties of water that contribute to its fundamental role in driving Earth Systems
- Identify U.S. and global precipitation patterns by reading precipitation maps
- Quantitatively compare fluxes of water in the hydrologic cycle
- Calculate relative humidity, and use it to quantitatively explain Earth's first-order patterns of precipitation
- Assess the relationship between precipitation, topography, and location on the globe
Unique Properties of Water
Unique Properties of Water
Water has some unusual properties that most of us do not really appreciate or understand. These properties are crucial to life and they originate from the structure of the water molecule itself. This sidebar will provide an overview of water's properties that will be useful in understanding the behavior of water in Earth's environment.
The Configuration of the Water Molecule
The Configuration of the Water Molecule
A molecule of water is composed of two atoms of hydrogen and one atom of oxygen. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. The electron ring of hydrogen would actually prefer to possess two electrons to create a stable configuration. Oxygen, on the other hand, has two electron rings with an inner ring having 2 electrons, which is cool because that is a stable configuration. The outer ring, on the other hand, has 6 electrons but it would like to have 2 more because, in the second electron ring, 8 electrons is the stable configuration. To balance the negative charge of 8 (2+6) electrons, the oxygen nucleus has 8 protons. Hydrogen and oxygen would like to have stable electron configurations but do not as individual atoms. They can get out of this predicament if they agree to share electrons (a sort of an energy "treaty"). So, oxygen shares one of its outer electrons with each of two hydrogen atoms, and each of the two hydrogen atoms shares it's one and only electron with oxygen. This is called a covalent bond. Each hydrogen atom thinks it has two electrons, and the oxygen atom thinks that it has 8 outer electrons. Everybody's happy, no?
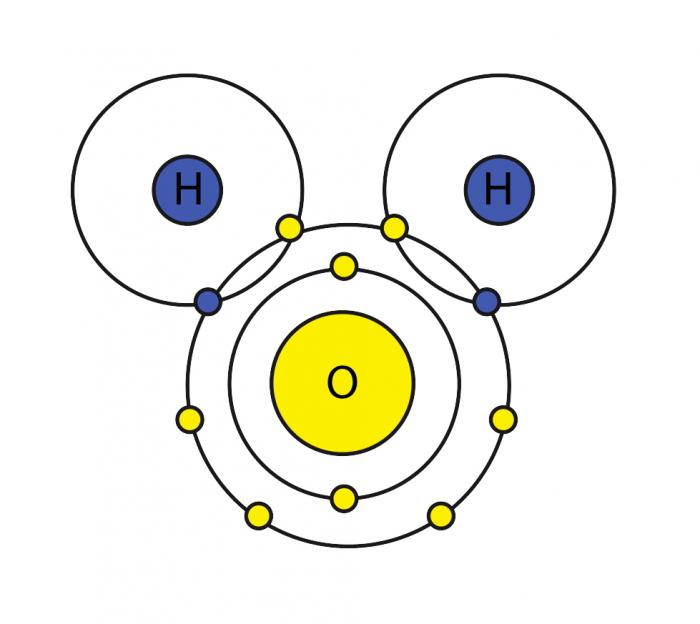
However, the two hydrogen atoms are both on the same side of the oxygen atom so that the positively charged nuclei of the hydrogen atoms are left exposed, so to speak, leaving that end of the water molecule with a weak positive charge. Meanwhile, on the other side of the molecule, the excess electrons of the oxygen atom, give that end of the molecule a weak negative change. For this reason, a water molecule is called a "dipolar" molecule. Water is an example of a polar solvent (one of the best), capable of dissolving most other compounds because of the water molecule's unequal distribution of charge. In solution, the weak positively charged side of one water molecule will be attracted to the weak negatively charged side of another water molecule and the two molecules will be held together by what is called a weak hydrogen bond. At the temperature range of seawater, the weak hydrogen bonds are constantly being broken and re-formed. This gives water some structure but allows the molecules to slide over each other easily, making it a liquid.
The Structure of Water: Properties
The Structure of Water: Properties
Studies have shown that clustering of water molecules occurs in solutions because of so-called hydrogen bonds (weak interaction), which are about 10% of the covalent water bond strength. This is not inconsiderable and energy is required to break the bonds, or is yielded by the formation of hydrogen bonds. Such bonds are not permanent and there is constant breaking and reforming of bonds, which are estimated to last a few trillionths of a second. Nonetheless, a high proportion of water molecules are bonded at any instant in a solution. But this structure leads to the other important properties of water.
We will consider, for the purposes of this course, only six of these important properties:
- Heat capacity
- Latent heat (of fusion and evaporation)
- Thermal expansion and density
- Surface Tension
- Freezing and Boiling Points
- Solvent properties
As mentioned above, these properties have importance to physical and biological processes on Earth. Effectively, large amounts of water buffer Earth surface environmental changes, meaning that changes in Earth-surface temperature, for example, are relatively minor. Thus, the high heat capacity of water promotes continuity of life on Earth because water cools/ warms slowly relative to land, aiding in heat retention and transport, minimizing extremes in temperature, and helping to maintain uniform body temperatures in organisms. However, there are other effects of water properties as well. Its low viscosity allows rapid flow to equalize pressure differences. Its high surface tension allows wind energy transmission to sea surface promoting downward mixing of oxygen in large water bodies such as the ocean. In addition, this high surface tension helps individual cells in organisms hold their shape and controls drop behavior (have you seen "An Ant's Life"?). Also, the high latent heat of evaporation is very important in heat/water transfer within the atmosphere and is a significant component of transfer of heat from low latitudes, where solar energy influx is more intense to high latitudes that experience solar energy deficits.
Video: Water - Liquid Awesome: Crash Course Biology #2 (11:16)
Take a few minutes to learn why water is the most fascinating and important substance in the universe.
Heat Capacity
Heat Capacity
Water does not give up or take up heat very easily. Therefore, it is said to have a high heat capacity. In Colorado, it is common to have a difference of 20˚ C between day and night temperatures. At the same time, the temperature of a lake would hardly change at all. This property originates because energy is absorbed by water as molecules are broken apart or is released by molecules of water associating as clusters.
Video: Heat Capacity of Water (01:13)
Take a few minutes to watch the video below to help you understand heat capacity.
Latent Heat and Freezing and Boiling Points
Latent Heat and Freezing and Boiling Points
A calorie is the amount of heat it takes to raise the temperature of 1 gram (0.001 liters) of pure water 1 degree C at sea level. It takes 100 calories to heat 1 g. water from 0˚, the freezing point of water, to 100˚ C, the boiling point. However, 540 calories of energy are required to convert that 1 g of water at 100˚ C to 1 g of water vapor at 100˚ C. This is called the latent heat of vaporization. On the other hand, you would have to remove 80 calories from 1 g of pure water at the freezing point, 0˚ C, to convert it to 1 g of ice at 0˚ C. This is called the latent heat of fusion.
Interestingly, the latent heat and freezing and boiling points are controlled by the way water molecules interact with one another. Because molecules acquire more energy as they warm, the association of water molecules as clusters begins to break up as heat is added. In other words, the energy is absorbed by the fluid and molecules begin to dissociate from one another. Considerable energy is required to break up the water molecule clusters, thus there is relatively little temperature change of the fluid for a given amount of heating (this is the heat capacity measure), and, even at the boiling point, it takes far more energy to liberate water molecules as a vapor (parting them from one another). On the other hand, when energy is removed from water during cooling the molecules of water begin to coalesce into clusters and this process adds energy to the mix, thus offsetting the cooling somewhat.
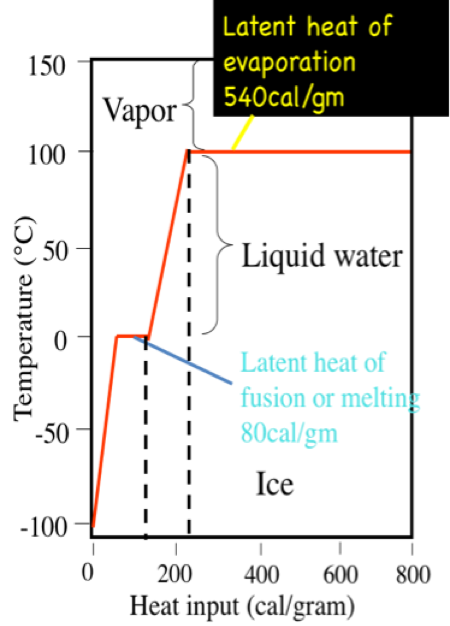
Click here for a text description
Thermal Expansion and Density
Thermal Expansion and Density
When water is a liquid, the water molecules are packed relatively close together but can slide past each other and move around freely (as stated earlier, that makes it a liquid). Pure water has a density of 1.000 g/cm3 at 4˚ C. As the temperature increases or decreases from 4˚ C, the density of water decreases. In fact, if you measure the temperature of the deep water in large, temperate-latitude (e.g., the latitude of PA and NY) lakes that freeze over in the winter (such as the Great Lakes), you will find that the temperature is 4˚ C; that is because fresh water is at its maximum density at that temperature, and as surface waters cool off in the Fall and early Winter, the lakes overturn and fill up with 4˚ C water.

However, as dissolved solids are added to pure water to increase the salinity, the density increases. The density of average seawater with a salinity of 35 o/oo (35 g/kg) and at 4˚ C is 1.028 g/cm3 as compared to 1.000g/cm3 for pure water. As you add salts to seawater, you also change some other properties. Incidentally, increasing salinity increases the boiling point and decreases the freezing point. Normal seawater freezes at -2˚ C, 2˚ C colder than pure water. Increasing salinity also lowers the temperature of maximum density. This effect also helps explain why you are supposed to add salt to ice when making ice cream or to add salt to water when cooking spaghetti (although, in this case, the effect on boiling point is minor and the added salt is mainly for flavor).
When water freezes, however, bonds are formed that lock the molecules in place in a regular (hexagonal) pattern. For nearly every known chemical compound, the molecules are held closer together (bonded) in the solid state (e.g., in mineral form or ice) than in the liquid state. Water, however, is unique in that it bonds in such a way that the molecules are held farther apart in the solid form (ice) than in the liquid. Water expands when it freezes making it less dense than the water from which it freezes. In fact, its volume is a little over 9% greater (or density ca. 9% lower) than in the liquid state. For this reason, ice floats on the water (like an ice cube in a glass of water). This latter property is very important for organisms in the oceans and/or freshwater lakes. For example, fish in a pond survive the winter because ice forms on top of a pond (it floats) and effectively insulates (does not conduct heat from the pond to the atmosphere as efficiently) the rest of the pond below, preventing it from freezing from top to bottom (or bottom to top).
If water did not expand when freezing, then it would be denser than liquid water when it froze; therefore it would sink and fill lakes or the ocean from bottom to top. Once the oceans filled with ice, life there would not be possible. We are all aware that expansion of liquid water to ice exerts a tremendous force. Have you or a family member (you wouldn't admit to this would you?) ever left a full container of water with a tight-fitting lid (or even a can of soda?) in the freezer? In other words, 10 cups of water put into the freezer is going to turn into 11 cups of ice when it freezes (oops). The force of crystallization of ice is capable of bursting water pipes and causes expansions of cracks in rocks, thus accelerating the erosion of mountains!
A rough sketch of water molecules in ice crystal form is below.

Surface Tension
Surface Tension
Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased to a very fine bore, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. This phenomenon is known as capillarity. This is a key property that allows trees to stand high, for example, because surface tension stiffens stems and trunks. Plants "wilt" because they are unable to acquire sufficient water to maintain the required surface tension. And, of course, water droplets (rain) and fog condensing as droplets on surfaces are a function of water's surface tension. Without this property, water would be a slimy coating and cells would not have shape. Surface tension decreases with temperature and salinity.
Video: Amusing Surface Tension Experiment (02:39)
Please take a few minutes to watch this amusing video to learn more about the surface tension of water.
The Universal Solvent
The Universal Solvent
This is, of course, another key property of water because more substances dissolve in water than any other common liquid. This is because the polar water molecule enhances "Dissolving Power." Dissolution involves breaking "salts" into component "ions." For example, NaCl (common salt) breaks down into the ions Na+ and Cl- because of the attraction for ions (atoms or groups of atoms with a charge) to water molecules is high.

Cations, such as Na (Sodium) have a net positive charge, whereas anions (such as Cl, Chloride) have a net negative charge. There are many individual elements and compounds that form ions. Thus, water can hold considerable concentrations of various chemical species depending on their particular properties. Note how the water molecules surround the individual ions, keeping them isolated from other ions in solution. This occurs until the capacity of water to isolate the ions is exceeded, at which point the solution is "saturated" with those ions and cannot dissolve more (salt will begin to precipitate—form a solid).
Learning Checkpoint
Water Distribution on Earth
Water Distribution on Earth
Where is water distributed on Earth?
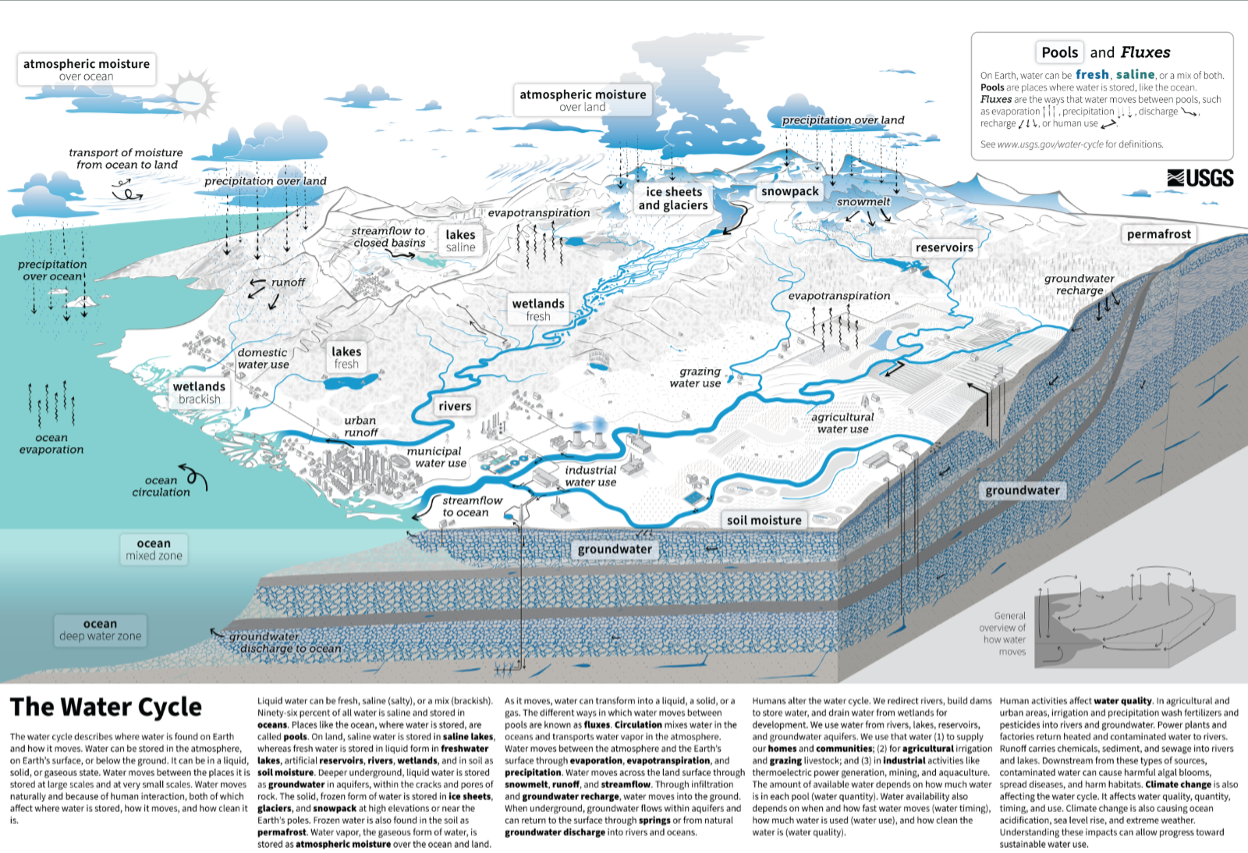
Earth is often called the “Blue Planet”, because of its abundance of liquid water. As we’ve already covered in Module #1, this water is distributed in the oceans, ice caps and glaciers, surface water (streams, lakes, and rivers), groundwater, soil moisture, the atmosphere, and in biomass. However, these reservoirs of Earth’s water are not static; water is constantly fluxing between them. We see this transport of water every day, for example in the form of flowing rivers, rain and snow, and groundwater springs.
Systems Thinking and the Hydrologic Cycle
Systems Thinking and the Hydrologic Cycle
Throughout this course, we will be dealing with complex systems and “Systems Thinking”. What is Systems Thinking, you may ask? According to Peter Senge, author of The Fifth Discipline Fieldbook, “Systems thinking is a way of thinking about, and a language for describing and understanding, the forces and interrelationships that shape the behavior of systems”. Some systems are very complex, but all systems can be simplified to help understand the relationships between systems components. Systems can be "modeled" to help investigate their dynamics. We do not expect you to become system modelers, per se, but simple models can begin to help you understand how changes in one parameter might influence changes in another. Let's consider a simple system in which we have a bathtub, fed by a faucet, and drained at its lower level. We could diagram this simple system as follows…

In this system there is a reservoir (the bathtub), an input (the faucet), and an output (the drain). The relationships in this system are simple and, hopefully, intuitive. If you want to run water into the tub for a long time to keep it quite warm, but not have it run over, what are your choices? You could keep the drain closed and run a very slow trickle of warm water into the tub from the faucet, letting it fill gradually, or, you could fill the tub quickly to some level, then open the drain to allow water to leave the tub at the same rate as it is being added to prevent further rise in the water level. Cold water is more dense than warm, so perhaps cooler water would drain preferentially and this would keep the tub water warmer overall. You could also evaluate the time it would take to fill the tub, or drain it, knowing the tub volume (gallons), the maximum input rate through the faucet (gallons/minute), and the maximum drain rate (gallons/minute).
Learning Checkpoint
Let's try a couple of simple model calculations to get you thinking about systems dynamics. First, we should establish some volumes and rates for this simple system. The tub (reservoir) will hold 30 gallons of water. The input and output values are outlined below:
1) If the faucet (input) will supply 3 gallons of water per minute, and the drain is closed (no output), how long will it take to fill the tub to the brim with water if the tub is empty to begin with?
2) If the faucet supplies 3 gallons per minute, the tub is empty to begin with, but the drain allows 3 gallons per minute to leave the tub, how long will it take the tub to fill?
3) If the faucet supplies 3 gallons per minute, the tub is empty to begin with, and the drain allows 1 gallon per minute to escape, how long will it take to fill the tub?
Hydrologic Cycle
Hydrologic Cycle
The movement of water between these reservoirs, primarily driven by solar energy influx at the Earth’s surface, is known as the hydrologic cycle.

The hydrologic cycle is a conceptual model that describes the fluxes of water between the oceans, surface water bodies (lakes, rivers, and streams), groundwater in subsurface aquifers, the atmosphere, and the biosphere. One important aspect of the cycle is that no water is gained or lost: water moves between reservoirs but the total mass remains the same. Another way to say this is that the water that currently exists on Earth is the same water that has been here since the time the Earth formed. (Technically, there are small fluxes of water from the Earth’s interior to the surface and atmosphere through volcanism and venting, and small influxes of water from comets and debris, but these are negligible in comparison to the mass of water in the primary reservoirs shown above.)
Activate Your Learning
1. There are five processes that control the movement of water between reservoirs in the hydrologic cycle. Looking at Figure 6 above, what do you think they are? Name as many as you can.
The movement of water between reservoirs, or the “limbs” of the hydrologic cycle includes five primary processes:
- Evapo-transpiration: the movement of water from oceans or land to the atmosphere, through the combined processes of evaporation and transpiration. Evaporation and transpiration both involve a change in state, from liquid to vapor, which requires an input of energy. Evaporation is simply the change from liquid to vapor as a result of molecular motion, and is affected by temperature and ambient humidity. Transpiration is the movement of water to the atmosphere by plant respiration. In most terrestrial basins, transpiration is the dominant process by which water moves from the Earth’s surface to the atmosphere, whereas over lakes and the oceans, evaporation dominates.
- Precipitation: the movement of water from the atmosphere to the land surface or oceans, in the form of rain, snow, sleet, ice pellets, etc... Precipitation involves a change in state from vapor to liquid, known as condensation. This change in state releases heat energy. After precipitation falls on the land surface, it may flow into surface water bodies (lakes or streams), or percolate through soils and rock into the groundwater system.
- Runoff: the movement of water from the land surface to the oceans in streams or rivers.
- Infiltration: the percolation of water from the land surface or from surface water bodies through soils and into the subsurface. Water that infiltrates becomes part of the groundwater system, and is also known as groundwater recharge.
- Groundwater outflow, also known as subsea outflow: the seepage of water from the groundwater system directly into the oceans. The flux of groundwater outflow is the least constrained component of the hydrologic cycle, and is often estimated by balancing the other fluxes in the cycle.
Because the changes in state that accompany evaporation and precipitation also take in and release energy, the movement of water through the hydrologic cycle is paralleled by redistribution of heat and energy.
Uneven Distribution
Uneven Distribution
Why is water distributed unevenly across the Earth’s surface?
As you probably know, things are far more interesting than a hypothetical case of evenly distributed precipitation! Both precipitation and evaporation vary widely over the Earth’s surface. This unequal distribution of water on the planet drives a diversity in climate and ecosystems (or biomes); water availability for human life, industry, and agriculture; and is fundamentally and intimately tied to the history of politics, economics, food production, population dynamics, and conflict – both in the U.S. and globally.
The abundance of water in some areas and scarcity in others follows systematic and predictable patterns. As part of this module, we’ll explore the physical processes that shape the overall distribution of precipitation - and thus water resources.



Learning Checkpoint
Note: The questions below are not graded. They may show up as summative evaluation questions on mid-term or final exams.
1) Look at Figure 7 above. What is the annual mean precipitation in Southern Nevada?
- 32 in/yr
- greater than 80 in/yr
- 0 in/yr
- 4-8 in/yr
2) Look at Figure 7 above. What is the annual mean precipitation in Coastal Washington State?
- a. 32 in/yr
- greater than 80 in/yr
- 0 in/yr
- 4-8 in/yr
3) Why do you think Nevada and Eastern Oregon are deserts?
- a. They are far North of the equator.
- They are far from the ocean.
- They are in the rain shadow of mountains.
- They are subject to large annual temperature fluctuations.
- They are at high elevation.
4) Look at Figure 8. What do you think is the global pattern of precipitation?
- a. It rains most South of the equator.
- There is East-West "banding" of climate/precipitation.
- There is North-South "banding" of climate/precipitation.
- There is snow in the Southern Hemisphere year-round.
Note the contrasting patterns in the two images in Figure 8 above, based on global satellite coverage. Vegetation in the southern hemisphere, which has relatively more ocean area (and less land area) than the northern hemisphere, changes little seasonally, whereas vegetation distribution in the northern hemisphere undergoes large changes. Why is that? There are probably two impacts on vegetation distribution—precipitation and temperature. Examine the figure below that illustrates the available moisture seasonally (summer vs. winter) and compare to the distribution of vegetation for the same seasons. Think about the role of temperature, precipitation, and soil moisture (water availability to plants), as well as the availability of sunlight for photosynthesis. Yes, there is a more complex relationship between plant growth and other factors, but the hydrologic cycle plays a major role.


Relative Humidity
Relative Humidity
The explanation for spatial variations in precipitation centers on the concept of relative humidity. The relative humidity is the water vapor pressure (numerator) divided by the equilibrium vapor pressure (denomator) times 100%. The equilibrium vapor pressure occurs when there is an equal (thus the word equilibrium) flow of water molecules arriving and leaving the condensed phase (the liquid or ice). Thus there is no net condensation or evaporation (Alistair Fraser, PSU).
Now, if the water vapor pressure is greater than the equilibrium value (numerator is greater), there is a net condensation (and a cloud could form, say). And that is not because the air cannot hold the water, but merely because there is a greater flow into the condensed phase than out of it.
Relative humidity describes the amount of water vapor actually in the air (numerator), relative to the maximum amount of water the air can possibly hold for a given temperature (denominator). It is expressed as a percentage:
If the relative humidity (RH) is 100%, this means that condensation would occur. On a typical hot muggy summer day, RH might be around 60-80%. In a desert, RH is commonly around 15-25%.

One important consequence is that when air masses change in temperature, the relative humidity can change, even if the actual amount of water vapor in the air does not (the numerator in our equation, which is defined by the saturation curve, stays the same, but the denominator changes with temperature). Figures 11-13 below show an example of this process. As the air cools, the relative humidity increases. If the air mass were cooled enough to become saturated (hit the solid black curved line), condensation would occur. This temperature is called the dew point.
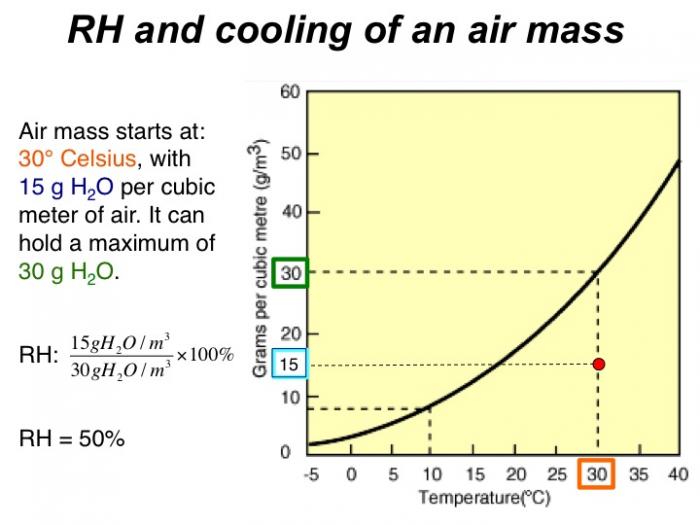


In the same way, changes in relative humidity occur when warm moist air is forced to rise or, conversely, when cool dry air descends. For example, when an air mass moves over mountains, it cools as it rises, and when it reaches the dewpoint, water will condense. This forms clouds, and if the air mass cools enough, the condensation becomes rapid enough to form precipitation.
The Orographic Effect
The Orographic Effect
To take the concept of relative humidity outdoors, let's consider why it rains in some areas and we have deserts in others. There are two primary reasons for this. Both are related to the transport, rise, and fall of air masses that lead to temperature changes, and ultimately in the amount of water vapor that the air can hold. These are the orographic effect, and atmospheric convection.
In both cases, cooling and warming of air masses occurs because they are forced upward or downward in the atmosphere. The decrease in air temperature with elevation is known as the atmospheric (or adiabatic) lapse rate, as shown below, and is related to decreasing air density and pressure with increasing altitude (as air rises, it expands due to decreased pressure, leading to lower temperature). A typical average lapse rate is around 7° C per km of altitude change. If an air mass begins rising and has not reached the dewpoint temperature, it follows a dry adiabatic lapse rate, with the rate of cooling due nearly entirely to decreasing pressure, as shown in Figure 14. Once the airmass temperature reaches the dewpoint during continued rise, water droplets begin to condense (forming clouds) and the airmass follows a moist adiabatic lapse rate (Figure 14), for which the rate of cooling with elevation decreases because of the addition of some offsetting heat to the airmass from the process of condensation (termed latent heat).

The orographic effect occurs when air masses are forced to flow over high topography. As air rises over mountains, it cools and water vapor condenses. As a result, it is common for rain to be concentrated on the windward side of mountains, and for rainfall to increase with elevation in the direction of storm tracks. With continued cooling past the dewpoint, the amount of water vapor in the air cannot exceed saturation, so water is lost from the air via condensation and precipitation.
On the leeward side of mountain ranges, the opposite occurs: the air descends and warms. As it does so, it is capable of holding more water vapor (recall the saturation line in the relative humidity plot above). However, there is no source of additional water, so the descending air mass increases in temperature but the amount of water vapor remains constant. Because the air has lost much of its original water content, as it descends and warms its relative humidity decreases. These areas are called rain shadows and are commonly deserts. You’ve probably noticed this same process in action when you heat your house or apartment in the winter – warming the cold air leads to dry conditions – one of the reasons people often put water pots or kettles on their wood stoves.
Orographic Effect In Action
The animation below shows an airmass trajectory superimposed on a Google Earth image of western North America. The point of this animation is to provide an explanation of the orographic effect and the changes in temperature and water content of an airmass passing over several mountain ranges. The animation shows the "rain shadow" effect that results in desert regions behind large mountain ranges. An inset graph at bottom right illustrates combinations of temperature (x-axis) and moisture content (y-axis) in grams per cubic meter of the air mass as it passes over various topographic features on the land surface.

Atmospheric Convection: Hadley Cells
Atmospheric Convection: Hadley Cells
There is a second, larger-scale effect that also plays a key role in the global distribution of precipitation and evaporation. Fundamentally, these patterns are also explained by the rise and fall, and cooling and warming of air masses – as is the case with the orographic effect – but in this case, their movement is a result of atmospheric convection rather than transport over topographic features.
As you have seen, there are regular climate and precipitation bands on the Earth – latitudes where most of the Earth’s tropical and temperature rainforests, deserts, polar deserts (also known as tundra) tend to occur. This global pattern – along with prevailing global wind patterns and storm tracks, are driven by atmospheric convection.
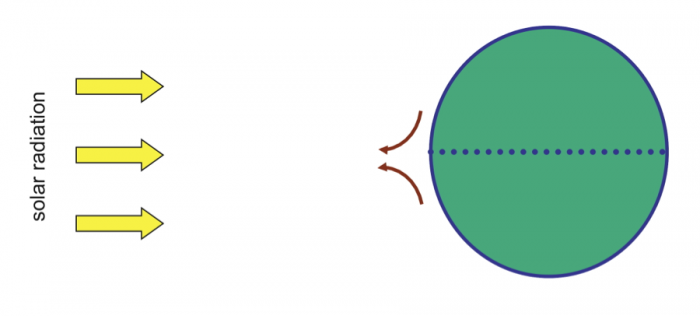
It all starts with solar radiation. Because of the Earth’s curvature, the tropics (between 23.5° N and S latitude) receive a larger flux of solar radiation per unit area on average than higher latitudes. Because the Earth’s axis is tilted, during Northern hemisphere summer, the peak influx of solar radiation occurs at 23.5° N latitude. During the Southern hemisphere summer, the maximum occurs at 23.5° S. (Incidentally, these latitudes define the tropics of Cancer and Capricorn.) Annually, the highest flux of solar energy per unit area occurs at the equator, as shown below.
As a result, the air around the equator becomes warmest. It holds quite a bit of water, too – based on the fact that, as you’ve seen above, warm air has a higher capacity to carry moisture.
Video Review: Global Atmospheric Circulation (2:24)
Take a few minutes to review the video below to help you understand Global Circulation a little better.
Energy Balance
Energy Balance




The differential heat input from solar radiation input and loss by infrared radiation is a critical part of maintaining equability (relatively low gradients in temperature from low to high latitudes) on the Earth. The energy balance figures indicate that above about 40 degrees North and South (e.g., the latitude of New York City) of the equator the loss of heat by radiation (infrared), on average, exceeds the input of heat from the sun (visible). What does that imply for our climate? One might think that this should result in permanent snow or ice above this latitude. Right? Indeed, during the last glacial epoch, about 21 thousand years ago, thick continental ice sheets extended to nearly 40 degrees North in North America (just north of I-80). But normally, because of the heat gradient created by the imbalance between solar input and infrared radiation, the atmosphere (and ocean) is set in motion to redistribute heat from low to high latitudes. Otherwise, the tropics would be excessively hot and the high latitudes excessively cold—at all times. Next, we will see how this circulation works.
Global Wind
Global Wind
As this warm air rises due to its lower density, it cools. Once it cools past the dewpoint, condensation occurs and clouds form. With continued rise and cooling, the air cannot hold the moisture and precipitation falls.
In response to that rising air, surface air must flow in to fill the vacated space. The rising air results in a low-pressure center. This is why when you hear about low pressure in the forecast, is typically associated with rising air masses and therefore with crummy weather. The air rushing in toward the equator defines the trade winds. These winds converge on the equator but blow to the West because of Earth’s rotation. This rotational effect is known as the Coriolis effect. We won’t get into that in detail here, but if you are interested, check out the video below.
Video: The Coriolis Effect (02:43)
These flows drive convection cells, with dimensions that are controlled by the viscosity and density of air, and by the thickness of the atmosphere. The air that rose from the equator flows North and South at the top of the cell and eventually descends at around 30° N or S latitude. As the cool, now dry air descends, it warms. Sound familiar?
Just as occurs when air descends on the leeward side of mountain ranges and causes rain shadows, the amount of water that the descending and warming air could hold increases. But there is no additional moisture to be found, so the actual amount of water vapor in the air mass remains more-or-less fixed. These descending limbs of the Hadley cells form high-pressure centers and would be regions where persistent dry conditions should prevail – leading to the Earth’s desert belts that include the Gobi, Sahara, Arabian, and the Australian Outback (not just a steakhouse!).
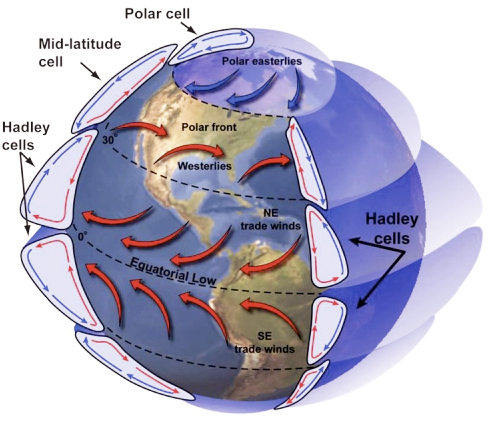
The equatorial convection cells are known as Hadley Cells. There are two more in each hemisphere, also driven by the uneven distribution of incoming solar radiation density; these are Farrell and Polar cells. Check out the diagram of this process below.

Global Wind Explained
Global Wind Explained
The illustration below portrays the global wind belts, three in each hemisphere. Note that the U.S. lies primarily in the Westerly Wind Belt with prevailing winds from the west. Each of these wind belts represents a "cell" that circulates air through the atmosphere from the surface to high altitudes and back again. The cells on either side of the Equator are called Hadley cells and give rise to the Trade Winds at Earth's surface. How do we explain this pattern of global winds and how does it influence precipitation?
We'll start at Earth's equator, where solar radiation is the highest year around. Air near the equator is warmed and rises because it is less dense (mass/unit volume) than the air around it as shown in Figure 21 below.
The rising air creates a circulation cell, called a Hadley Cell, in which the air rises and cools at high altitudes moves outward (towards the poles) and, eventually, descends back to the surface. The continual heating and rise of air at the equator create low pressure there, which causes air to move (wind) towards the equator to take the place of the air that rises. On the other hand, sinking air creates high pressure at the surface where it descends. A gradient of pressure (high to low) is formed that causes air to flow away from the high and towards the low pressure at the surface.


The Earth would have two large Hadley cells if it did not rotate. But, because it does rotate, the rotation of the Earth leads to the Coriolis effect. You should view the short video on this so-called "effect" or "force." (The Coriolis Effect [6]). Without going into detail as to why rotation creates this apparent force, the Coriolis effect causes winds (and all moving objects) to be deflected:
- to the right in the Northern Hemisphere
- to the left in the Southern Hemisphere
The Coriolis effect causes winds to deflect as they travel within circulation cells and results in the two large hypothetical Hadley cells breaking into six smaller cells, which looks something like the diagram below (and the first figure in this series).

Ok, so, we now have some idea about the origin of global wind systems that result from pressure gradients at Earth's surface. How does this produce precipitation, and where? Precipitation occurs where moisture-laden air rises, either by heating at the equator or by running up and over a more dense air mass. As the rising air cools its capacity to hold water decreases (relative humidity increases) and, at some point, saturation with respect to water vapor is reached. Then, condensation--clouds and rain!

The diagrams above and below portray just the Hadley cell circulation, that is driven by heating in the equatorial region. On the surface, wind moves away from high pressure (High) and toward low pressure (Low). Convergence occurs near the equator (winds blow in towards one another) and Divergence occurs under the descending air that forms high-pressure belts. The final figure (Figure 26) shows all six cells diagrammatically, along with the pressure variations at the surface of the Earth and zones of typical wet and dry belts. Note particularly the dry belts near 30 degrees North and South.