Lessons
Lesson 1: Energy and Society
The links below provide an outline of the material for this lesson. Be sure to carefully read through the entire lesson before returning to Canvas to submit your assignments.
Introduction & Checklist
Welcome to Lesson 1!
It looks like we are ready to go for lesson 1: Energy and Society. This lesson is going to teach us about energy! What is energy, and with which units do we measure energy? We will learn about the commonly used units we use to measure energy. There are different forms of energy that we use; for example, we use electrical energy or mechanical energy, like moving a car, etc., and we need to know the units in which we measure these different forms of energy. So we will also learn about forms of energy. We will learn about the units in which we measure these forms of energy, and also we will get into a very, very important distinction between energy and power. To be clear, that is the key concept in this lesson that I want you to concentrate on – power and energy. And once we know the difference, we know that using power, we can calculate energy, or if we know the energy and time, we can calculate power.
We will also look at some of those calculations. Once we know the power, we can calculate the energy. For example, a computer consumes some power, the rate at which energy is drawn, and if we use the computer for so many hours, what is the energy consumption by this computer? We can do the same thing for a refrigerator, or we can do it for any other appliance that you use at home. These are common appliances that we are using every day in our lives. When we add up the energy consumed by a computer, by a toaster, by an oven, by a refrigerator, by lighting, etc., at your place then you get energy consumption of all your equipment for a day. So we are going to do that and calculate energy consumption for a day, and then for a month, and we can calculate also the electric bill for one whole month -- that would be our objective in this lesson.
Be careful, again, because the distinction between energy and power is a very important concept. Forms of energy and the units in which we measure energy are the concepts that we will be looking at in this wonderful lesson. All Right! Why Wait? Let’s go and start our lesson.
Good Luck!
What is Energy?
When thinking about energy the following questions may come to mind:
- What is energy?
- How do we measure it?
- Where is it coming from?
- Do we have enough?
- What is the impact of energy use?
Energy is the lifeblood of any modern society. Energy is used in every walk of life. Without it, modern life would almost come to a standstill. From the moment of waking up in the morning with an alarm clock, we use energy for almost everything we do.
Energy is a property of matter that can be converted to work, heat, or radiation. It can move things or do work, produce heat even if it does not move anything, and be converted to light (or more accurately, radiation).
Lesson 1 Objectives
Upon completing this lesson, you should be able to:
- define energy
- articulate fundamental forms of energy
- know the different units of energy
- define and distinguish differences between energy and power
- classify Energy Sources
Checklist for Lesson 1
| Step | Activity | Access / Directions |
|---|---|---|
| 1 | Read the online lesson | Lesson 1 - Energy Supply and Demand |
| 2 | Review | Lesson 1 - Review & Extra Resources (supplemental materials that are optional...but informative!) |
| 3 | Take | Lesson 1 - Quiz (graded) The quiz is available in Canvas. |
Please refer to the Calendar in Canvas for specific timeframes and due dates.
Questions?
If you have any questions, please post them to the General Course Questions forum in located in the Discussions tab in Canvas. I will check that discussion forum daily to respond. While you are visiting the discussion board, feel free to post your own responses to questions posted by others - this way, you might help a classmate!
Forms of Energy, page 1 of 2
Six Basic Forms of Energy
Energy exists in a number of different forms, all of which measure the ability of an object or system to do work on another object or system. There are six different basic forms in which we use energy in our day-to-day life:
Mechanical Energy (Kinetic)
- Energy that a body possesses by virtue of its motion. A few examples are a baseball player pitching a ball, a plow being pulled by a tractor, and a hammer that is being used to pound nails.
- In the United States, we use about a third of our total energy for transportation or movement of people and goods.
Mechanical Energy (Potential)
- Energy that a body possesses by virtue of its position relative to a reference point. A few examples of mechanical energy include a pendulum, a bow (archery), a spring, and a hammer that is raised in preparation to pound nails.
Let's investigate further...
A book sitting on a shelf in the library is said to have potential energy because if it is nudged off the shelf, gravity will accelerate the book, giving the book kinetic energy. Because the Earth's gravity is necessary to create this kinetic energy, and because this gravity depends on the Earth being present, we say that the Earth-book system is what really possesses this potential energy, and that this energy is converted into kinetic energy as the book falls.
Chemical Energy
- Energy locked in the bonds of molecules in the form of microscopic potential energy, which exists because of the electric and magnetic forces of attraction exerted between the different parts of each molecule.
- It is the same attractive force involved in thermal vibrations.
- The molecular parts get rearranged in the chemical reactions, releasing or adding to this potential energy.
- Some examples include a battery, burning wood, and glucose in the body.
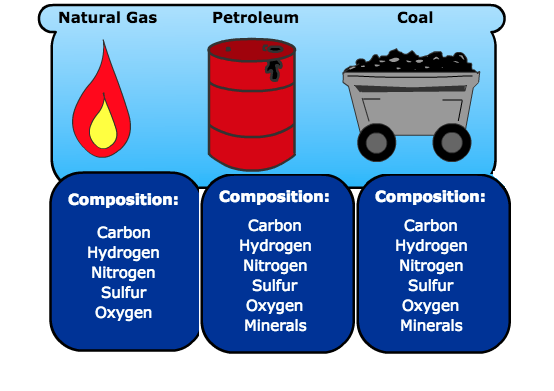
- As of 2020, approximately 80% of the energy used in the U.S. comes from fossil fuels such as coal, oil, and natural gas.
- All of these fuels store energy in the form of chemical energy.
- When they are burned, these fuels release energy in the form of heat or thermal energy.

The glucose (blood sugar) in your body is said to have "chemical energy" because the glucose releases energy when chemically reacted (combusted) with oxygen. Your muscles use this energy to generate mechanical force (work) and also heat.
Thermal or Heat Energy:
- Energy that combines microscopic, kinetic, and potential energy of the molecules. Some examples of this include a hot beverage and boiling water.
- Temperature is really a measure of how much thermal energy something has: The higher the temperature, the faster the molecules are moving around and/or vibrating, i.e., the more kinetic and potential energy the molecules have.
- Fuels (chemical energy) are oftentimes burned and converted to thermal or heat energy, which is then converted to motion in an automobile or electricity.
Thermal Energy
A hot cup of coffee is said to possess "thermal energy," or "heat energy," because it has a combination of kinetic energy (its molecules are moving and vibrating) and potential energy (the molecules have a mutual attraction for one another) - much the same way that the book on the bookshelf and the Earth have potential energy because they attract each other.
Forms of Energy, page 2 of 2
Electrical Energy:
- Energy created through the movement of electrons among the atoms of matter.
- Although electricity is seldom used directly, it is one of the most useful and versatile forms of energy. Following are some examples. When electricity is:
- put into a toaster, it can be converted to heat;
- put into a stereo, it is converted into sound;
- put into an electric bulb, it converts into light;
- put into a motor, it converts into motion or movement (mechanical energy).
- Due to its versatility, electricity is in high demand; in the US, about 40% of the total primary energy used is converted into electricity for various uses.
Remember This!
All matter is made up of atoms, and atoms are made up of smaller particles called protons (which have positive charge), neutrons (which have neutral charge), and electrons (which are negatively charged).
- The electrons orbit around the nucleus (which contains protons and neutrons), just like the planets orbit the sun.
- Certain metals have electrons that are only loosely attached to their atoms, so they can be easily made to move from one atom to another if an electric field is applied to them.
- When those electrons move among the atoms of matter, a current of electricity is created.
Nuclear Energy:
- Energy produced when reactions occur in an atom, resulting in some type of structural change in the nuclei.
- Fusion occurs when two small nuclei join together to create one large nucleus or particle, and during this process, energy is released in the form of light and heat. An example is in the Sun: hydrogen nuclei fuse (combine) together to make helium nuclei, which release energy.
- Fission occurs when the nucleus of one big atom splits into two new atoms, and during this process, a tremendous amount of energy is released in the form of light and heat. An example is in a nuclear reactor or the interior of the earth: uranium nuclei split apart, causing energy to be released.
Did You Know?
In both fusion and fission, some of the matter making up the nuclei is converted into energy, represented by the famous equation:
- This formula indicates that energy intrinsically stored in matter at rest equals its mass times the speed of light squared. When matter is destroyed, the energy stored is released.
- This equation suggests that an incredibly huge amount of energy is released when a small amount of matter is converted to energy.
Radiation:
- Energy radiated or transmitted in the form of rays, waves, or particles. Some examples include:
- visible light that can be seen by naked eye;
- infrared radiation;
- ultraviolet radiation (UV) that cannot be seen with the naked eye;
- long wave radiation, such as TV waves and radio waves;
- very short waves, such as x-rays and gamma rays.
Even things that we encounter in our every day life contain some radioactive material, either natural or man-made. Smoke detectors, compact fluorescent bulbs, some watches and granite countertops can emit some nuclear radiation. Even plane travel at high altitudes cause exposure from cosmic rays.
- Electromagnetic Radiation
- Energy from the sun comes to the earth in the form of Electromagnetic radiation, which is a type of energy that oscillates (side to side) and is coupled with electric and magnetic fields that travel freely through space.
- Electromagnetic radiation is composed of photons or particles of light, which are sometimes referred to as packets of energy.
- Photons, like all particles, have properties of waves.
Photons make the world a brighter place!
Photons are created when electrons jump to lower energy levels in atoms, and are absorbed when electrons jump to higher levels. Photons are also created when a charged particle, such as an electron or proton, is accelerated. An example of this phenomenon is a radio transmitter antenna that generates radio waves.
- Electromagnetic Spectrum
- The “Electromagnetic spectrum“ is a representation of the wide range of wavelengths of electromagnetic radiation.
- Photons are associated with visible light, which accounts for only a very limited part of the electromagnetic spectrum.
- A great discovery of the nineteenth century was that radio waves, x-rays, and gamma-rays are just forms of light, and that light is electromagnetic waves.
Please watch the following 5:00 video about the electromagnetic spectrum:
Something surrounds you. Bombards you. Some of which you can't see, touch, or even feel. Every day, everywhere you go. It is odorless and tasteless. Yet you use it and depend on it every hour of every day. Without it the world you know could not exist. What is it? Electromagnetic radiation. These waves spread across the spectrum from very short gamma rays to x-rays, ultraviolet rays, visible light rays, even longer infrared light waves, microwaves, to radio waves which can measure longer than a mountain range. This spectrum is the foundation of the information age and of our modern world. Your radio, remote control, text message, television, microwave oven, even a doctor's x-ray, all depend on waves within the electromagnetic spectrum.
Electromagnetic waves, or EM waves, are similar to ocean waves in that both are energy waves. They transmit energy. EM waves are produced by the vibration of charged particles and have electrical and magnetic properties. But unlike ocean waves that require water, EM waves travel through the vacuum of space at the constant speed of light. EM waves have crests and troughs like ocean waves. The distance between crests is the wavelength. While some EM wavelengths are very long and are measured in meters, many are tiny and are measured in billionths of a meter, nanometers. The number of these crests that pass a given point within one second is described as the frequency of the wave. One wave or cycle per second is called a Hertz. Long EM waves, such as radio waves, have the lowest frequency and carry less energy. Adding energy increases the frequency of the wave and makes the wavelength shorter. Gamma rays are the shortest, highest energy waves in the spectrum. So, as you sit watching TV, not only are there visible light waves from the TV striking your eyes, but also radio waves transmitting from a nearby station; and microwaves carrying cellphone calls and text messages; and waves from your neighbors Wi-Fi and GPS units in the cars driving by. There's a chaos of waves from all across the spectrum passing through your room right now.
With all of these waves around you, how can you possibly watch your TV show. Similar to tuning a radio to a specific radio station, our eyes are tuned to a specific region of the EM spectrum and can detect energy with wavelengths from 400 to 700 nanometers. The visible light region of the spectrum. Objects appear to have color because EM waves interact with their molecules. Some wavelengths in the visible spectrum are reflected and other wavelengths are absorbed. This leaf looks green because EM waves interact with the chlorophyl molecules. Waves between 492 and 577 nanometers in length are reflected and our eye interprets this as the leaf being green. Our eyes see the leaf as green but cannot tell us anything about how the leaf reflects ultraviolet, microwave, or infrared waves.
To learn more about the world around us, scientists and engineers have devised ways to enable us to see beyond that sliver of the EM spectrum called visible light. Data from multiple wavelengths help scientists study all kinds of amazing phenomena on Earth from seasonal change to specific habitats. Everything around us emits, reflects, and absorbs EM radiation differently based on it's composition. A graph across the EM spectrum is called the spectral signature. Characteristic patterns like fingerprints within the spectra allow scientists to determine an object's chemical composition and to determine such physical properties as temperature and density.
NASA's Spitzer space telescope observed the presence of water and organic molecules in a galaxy 3.2 billion light years away. Viewing our sun in multiple wavelengths with the SOHO satellite allows scientists to study and understand sunspots that are associated with solar flares and eruptions that are harmful to satellites, astronauts and communications here on Earth.
We are constantly learning more about our world and universe by taking advantage of the unique information contained in the different waves across the EM spectrum.
As depicted in the image above, the lower the energy, the longer the wavelength and lower the frequency, and vice versa.
The reason that sunlight can hurt your skin or your eyes is because it contains "ultraviolet light," which consists of high energy photons. These photons have short wavelength and high frequency, and pack enough energy in each photon to cause physical damage to your skin if they get past the outer layer of skin or the lens in your eye.
Radio waves, and the radiant heat you feel at a distance from a campfire, for example, are also forms of electromagnetic radiation, or light, except that they consist of low energy photons (long wavelength and high frequencies - in the infrared band and lower) that your eyes can't perceive. This was a great discovery of the nineteenth century - that radio waves, x-rays, and gamma-rays are just forms of light, and that light is electromagnetic waves.
About 20% of the electricity used in the US is used to produce visible light for lighting purposes.
Activity: Identifying Forms of Energy
Can you identify the different forms of energy in the picture below? Enter your answer in the table below and click the "Check Answers" button to check your work.
These things, listed below, represent the six fundamental forms of energy: Mechanical, Chemical, Thermal/Heat, Electrical, Nuclear and Radiation. Your task is to determine what form of energy is represented by each item.
- Light bulb in a lamp post powered by?
- Two women sitting at a picnic table drinking water. The arrow is pointing to the cups of water. One cup is sitting on the table and the other is in a woman's hand.
- A doctor looking at an X-ray produced with?
- A Frisbee flying through the air powered by?
- The sun power by?
- A man getting ready to hit a golf ball with a golf club. The arrow is pointing at the head of the golf club which is powered by?
- A little boy eating an ice cream cone. The arrow is pointing to the ice cream that provided what to the child?
Now spend some time trying to identify the different forms of energy that are at work in the above items. Once you have thought through this and have some answers, read on to see if you are correct.
AnswersLight bulb in a lamp post - Electrical Energy
Cups of water. One is sitting on a table and the other is in a woman's hand. - Thermal or Heat Energy
An X-ray - Nuclear Energy
A Frisbee flying through the air - Mechanical (kinetic) Energy
The sun - Radiation
A golf club getting ready to hit a ball - Mechanical (Potential)
The ice cream in an ice cream cone - Chemical
Energy Conversion
Energy can be converted from one form to another.
Examples:
- Gasoline (chemical) is put into our cars, and with the help of electrical energy from a battery, provides mechanical (kinetic) energy.
- Purchased electricity is fed into our TVs and is converted to light and sound.
- Similarly, purchased electricity goes into an electric bulb and is converted to visible light and heat energy.
- The image below shows examples of more conversions.
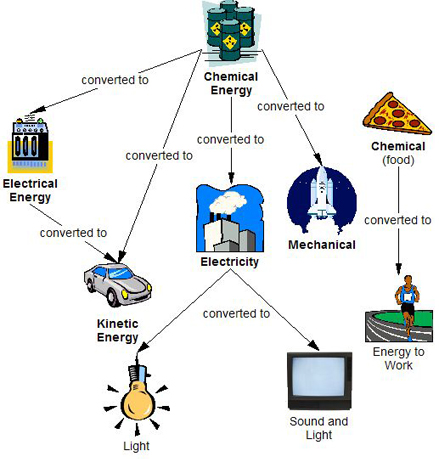
Chemical Energy is converted to Electrical Energy (stove), Kinetic Energy (car), Electricity (power plant), and Mechanical Energy (space shuttle). Electrical Energy is converted to Kinetic Energy. Electricity is converted to Light (light bulb) and Sound and Light (TV). Chemical food energy is converted to Energy to Work (person running).
Activity: Day-to-Day Conversion Devices
Activity: Day to Day Conversion Devices
Most of the day-to-day devices that we use are energy conversion devices. In this activity, you will identify the fundamental form of energy that is put in to each device and the output form of energy that is a result.
Your task is to look at six devices and decide what form of energy is the input and which is the output form of energy. Think about your answers carefully before reading ahead to the answers.
- Lawn Mower input form of energy? Output form of energy?
- Computer input form of energy? Output form of energy?
- Sun input form of energy? Output form of energy?
- Tree input form of energy? Output form of energy?
- Gas Furnace input form of energy? Output form of energy?
- Hair Dryer input form of energy? Output form of energy?
| Device | Input form of Energy | Output form of energy | |
|---|---|---|---|
| 1 | Lawn Mower | Chemical | Mechanical or kinetic |
| 2 | Computer | Electrical | Light and Sound |
| 3 | Sun | Nuclear | Radiant |
| 4 | Tree | Radiant | Chemical |
| 5 | Gas Furnace | Chemical | Thermal or Heat |
| 6 | Hair Dryer | Electrical | Heat or Electric |
Measurement of Energy
Units of Measurement
How is energy measured? It is measured in various units by various industries or countries, in much the same way as the value of goods is expressed in Dollars in the U.S. and Yen in Japan and Pounds in Britain.
The table below identifies different units for measuring energy. A lot of it also has some historical context. Our early studies of energy involved heating things up, so we name units based on how hard it was to heat things. Makes sense, right? Now we pass electrical energy to operate many devices, so now we use units that "better" capture this process.
| Unit | Definition | Used In | Equivalent to |
|---|---|---|---|
| British Thermal Unit BTU | A unit of energy equal to the amount of energy needed to raise the temperature of one pound of water by one degree Fahrenheit. Equivalent to energy found in the tip of a match stick. | Heating and Cooling industries | 1 BTU = 1,055 Joules (J) |
| calorie or small calorie (cal) | The amount of energy needed to raise the temperature of one gram of water by one degree Celsius. | Science and Engineering | 1 calorie = 0.003969 BTUs |
| Food Calorie, Kilocalorie or large calorie (Cal, kcal, Calorie) | The amount of energy needed to raise the temperature of one kilogram of water one degree Celsius. The food calorie is often used when measuring the energy content of food. | Nutrition | 1 Cal = 1,000 cal, 4,187 J or 3.969 BTUs |
| Joule (J) | It is a smaller quantity of energy than calorie and much smaller than a BTU. | Science and Engineering | 1 Joule = 0.2388 calories and 0.0009481 BTUs |
| Kilowatt Hour (kWh) | An amount of energy from the steady production or consumption of one kilowatt of power for a period of one hour. | Electrical fields | 1 kWh = 3,413 BTUs or 3,600,000 J |
| Therm | A unit describing the energy contained in natural gas. | Home heating appliances | 1 therm = 100,000 BTUs |
Did You Know?
When writing BTUs, one uses a base of “10” raised to a particular exponent.
For example:
More specific notation involves the following:
To express measurements greater than those with a base of 10, you would do the following:
Here is a fun way to understand your energy use
Prof. Bruce Logan of Penn State published a fascinating way to view your energy and climate impact. Using what you learned in this section, you can start to piece together just how much energy each of us uses to maintain our busy lifestyles.
The premise of this approach is to define (another!) unit of energy, but one with a bit more meaning. The daily energy unit, D. We are all supposed to eat about 2000 food Calories a day to survive. So, let’s set this amount of energy to equal 1 D. Now, how many Ds does the typical U.S. home each day (normally in KWh) or operate a car (normally joules or BTUs )? This method of comparing energy consumption allows us to better understand the scale of our energy habits (which might be shocking!) and tell you how many big mac-powered humans it would take to do what your car does…
Here are a few examples he shows to give you an idea.
- Food for 1 day = 1 D
- Running a single 100 W light bulb all day = 1.03 D
- Average daily electricity use for a US house = 13 D
- 1 gallon of gasoline used in an average car (goes 18 miles) = 15.2 D
- Natural gas for daily heating a US house = 31 D
Once we tally up all the energy it takes to fuel our lifestyle (professional + personal uses), each person consumed about 101 D of energy! (remember this is daily) For comparison, a Swiss citizen consumes about 54 D. Check out his website for more comparisons. Watch this video (5 min 46 sec)
Energy Literacy The daily energy unit "D"
By Bruce Logan Penn State University.
If we want to communicate we need to speak the same language. How can we do that, well we translate it into something we know, into one common language. If you want to communicate when it comes to energy, how do we do that. We translate it into something that we know. Something we all know is that we need about 2,000 calories a day to live. That's something we can relate to. But how do we relate that to other things that either consume energy or produce energy in our lives.
Well, for example, if I were to take that 2,000 calories a day that I eat and use that energy to power 100 watt light bulbs, how many could I power. 3 4 5 10? The answer is just one light bulb. A single light bulb running continuously consumes the equivalent of about 2,000 calories a day.
Now if you look at all the things that we encounter in our lives in terms of energy units, so many of them have different units. For example 2,000 calories with a capital C is really 2,000 kilocalories. Daily food for a horse is 20,000 kilocalories. The energy in a gallon of gasoline 114 thousand BTUs or British thermal units. Or maybe you look at the engine in your car and it's 120 horsepower.
How can we understand these units. Well, one way is we could put them all on the basis of a kilowatt hour. However, look at those numbers. They're all still rather confusing and it's tough to relate to a kilowatt hour. So we need to find something that's not quite so confusing.
I propose that we define 2,000 calories a day, or the food that one person eats, as the unit 1D. One daily energy unit. If we use that unit, we could say well, our home uses 13D. Or while we eat 1D, a horse needs to eat 10D. The energy in a gallon of gasoline is about 14D.
So this common unit of D, allows us to now compare all these things in terms of something that we know, that is how much food we need to stay alive. And that unit of 1D is the same to everybody on the planet. Some people need a little bit more some people need a little bit less, but the general concept of the food we eat every day can help define what energy consumption is like.
So when you look at things now, for example, you can see the electricity that we consume per capita, not necessarily just in your home, but averaged across to all people in the US, is about 40 D, and all energy normalized per person in the U.S. is about 104 D. That makes this unit of D very nice because it ranges for the food and other activities up to about a maximum of about a hundred.
Look at your car which say gets 18 miles per gallon and your commuting 18 miles. Your commute costs you about 14D, 1 gallon of gas. If you were to travel in a more fuel-efficient car, say a Toyota Prius or something like that, that commute might only take 4.9D because you would use less gasoline. Or what if you used an electric car. Well to charge that car and use the energy in that battery, it's about 27 kilowatt hours to go 100 miles or 2.1D.
Let's say that you want to put solar panels on your house. Well each solar panel produces 0.43D. That means averaged over the day and with typical sunlight, and for example Pennsylvania, you would achieve about 0.43 D of energy capture out of each one of those panels. So if your house uses 13D, well you need about 30 solar panels. If your car is an electric car and it uses 2.1 D, then your commute needs about 2.1D or about 5 or 6 solar panels.
How much energy should we use. Well there's a study done by ETH Zurich which suggests a 2,000 watt Society. 2,000 watts is about 20D. Currently, Switzerland has the average population having about 53 D which is about half of what we consume in the U.S., which is about 104D. So if we're gonna have to try and reduce our energy consumption that's gonna have to go down quite a lot.
Going forward, how can we do that. Well, can you reduce the energy for your commute. Can you save more energy at home or energy you say for transport or food or put a solar panels on your house and reduce your consumed D.
So the question really is, going forward, how can we make that 20D green.
Thanks for listening and I hope that you find a way to use D in your life to understand the energy you can see.
Sources of Energy
Energy is stored and is available in different forms and sources. The ~24,000 times more solar energy that is available than we need is not in a readily usable form. It needs to be concentrated.
For example, when oil (a concentrated fuel) is burned with air, the resulting gases can reach high temperatures. Solar energy, as it is, is not concentrated and cannot reach those high temperatures. Therefore, we use more concentrated energy sources. These sources are divided into two groups—renewable and nonrenewable.
Renewable Energy Sources:
- Energy sources that can be replenished over and over again; they are never depleted. Some examples include hydropower, solar, wind, tidal, geothermal energy from inside the earth, biomass from plants, and nuclear fusion.
- These types of energy sources are usually converted into electricity or thermal (heat) energy.
Nonrenewable Energy Sources:
- Energy sources that we are using up and cannot produce in a short period of time. Some examples include fossil fuels (Petroleum Oil, Natural Gas, and Coal), Tar Sands, and Nuclear Fission.
- Another nonrenewable energy source is the element uranium, whose atoms we split (through a process called nuclear fission) to create heat, and ultimately, electricity.
- These types of energy sources are usually converted into electricity and mechanical energy.
- We get most of our energy from these nonrenewable energy sources.
Did You Know?
They're called fossil fuels because they were formed over millions and millions of years by the action of heat from the Earth's core and pressure from rock and soil on the remains (or 'fossils') of dead plants and animals.
Fossil Fuel Distribution
Fossil fuels, non-renewable energy sources formed over a million years, are not distributed uniformly over the earth’s surface. Depending on the climate conditions millions of years ago, certain parts of the land masses were favorable for organic matter to grow and thrive.
Over geological ages, these land masses moved, and certain regions are richer in fossil fuels than others. Review the information on the map below, and then answer the questions below the map based on your observations.

Click link to expand for a text description of the image
Global Distribution of Natural Resources
The most abundant resources for various global regions are as follows:
- China is richest in coal
- Australia is richest in coal
- The Middle East is richest in petroleum
- The United States is richest in coal
- Russia is richest in natural gas
Human Use of Energy Sources
The activity below is a drag and drop. Select the items listed in the center of the image and drag them to the corresponding or matching energy type listed on the side.
Human use of Energy Sources
The following eight items are examples of how renewable and nonrenewable energy sources are used. Take some time to decide if each example is renewable or nonrenewable and then describe its specific source. (wind, sun, geothermal, water, oil, natural gas, uranium, or coal).
The answers will be at the bottom of the page.
- Nuclear power plant
- Gas stove
- Dam
- Gas pump
- Geothermal heat pump
- Power lines
- Solar panels
- Windmills
ANSWERS:
- Nuclear power plant is a nonrenewable energy source using uranium as its source.
- The gas stove is an example of nonrenewable energy source that uses natural gas as its source.
- A dam is an example of a renewable energy source that uses water as its source.
- A gas pump is an example of a nonrenewable energy source that uses petroleum as its source.
- A geothermal heat pump is a renewable energy source that uses geothermal heat (ground heat) as its source.
- Power lines are an example of a nonrenewable energy source that uses coal as its source.
- Solar panels are a renewable energy source that use the sun as their source.
- Windmills are a renewable energy source that use the wind as their source.
Power
What is Power?
Click the "play" button below and observe what happens. (Note: The video has no audio.)
Power vs. Energy
Both cyclists did the same amount of work (they both pedaled 10 miles), and used the same amount of energy (218 calories). The blue cyclist, however, demonstrated the most power, because he did the equivalent amount of work as the red cyclist, but in a faster time.
Power is the rate at which we do work.
Energy is the capacity to do work.
Work is the amount done.
Measuring Power
Units of Power are not the same as units of energy (i.e., Btus, calories). Units of power are measured in terms of units of energy used per some unit of time.
Examples of Units of Power include:
- Watt (W) = 1 joule of energy per second or 1 J/S
- BTU per hour (BTUs/h) = 1,055J
- Horsepower (hp) = 550 foot-pounds per second or 550 ft lb/S
- Calories per second (cal/sec)
- Kilowatt (kW) = 1,000 watts
Calculating Power
Power can be determined by the following formula:
Example
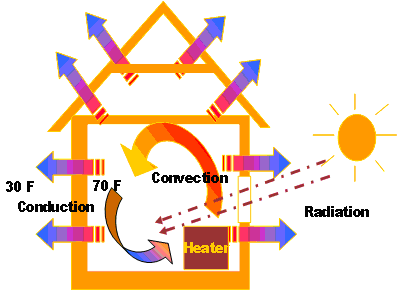
On a winter day, a home needs 1 x 106 or 1,000,000 BTUs of fuel energy every 24 hours to maintain the interior at 65° F. At what rate is the energy being consumed in Watts?
If 1 J/s = 1 Watt, and 1,000 Watt = 1kW, then 12,200 J/s = 12,200 Watts = 12.2 kW
 To solve this problem, you must realize the following: You know the Power (1,000,000 BTUs/24 hours) and the time (24 hours), so you need to solve for Energy. The measurements must be consistent, so the BTUs should be converted to a consistent measure, such as Joules:
To solve this problem, you must realize the following: You know the Power (1,000,000 BTUs/24 hours) and the time (24 hours), so you need to solve for Energy. The measurements must be consistent, so the BTUs should be converted to a consistent measure, such as Joules:
If using Joules per second instead of watts, you must convert 24 hours into seconds or divide it by the number of seconds in an hour (3,600).
Image Credit: © Penn State University, is licensed under CC BY-NX-SA 4.0
Power & Cost of Energy
We can also use a version of the Power formula to determine Cost of Energy:
Example
If a 100 W light bulb is accidentally left on overnight (8 hours), how much energy does it consume?
How much energy does this cost, if electricity costs 10 cents per Kilowatt?
Energy Use of Home Appliances, page 1
Calculating Energy Use
How is energy use of Home Appliances calculated?
You just learned in a previous discussion on power that:
or
By modifying this formula slightly, we can determine Energy Consumption per Day:
Where:
- Energy Consumption will be measured in Kilowatt hours (kWh) - like on your utility bills.
- Power Consumption will be measured in Watts
- Hours used per Day will be the actual time you use the appliance.
Since we want to measure Energy Consumption in Kilowatt hours, we must change the way Power Consumption is measured from Watts to Kilowatts (kWh). We know that 1 kilowatt hour (kWh) = 1,000 Watts hours, so we can adjust the formula above to:
Example 1: Calculating Energy Use of a Ceiling Fan
If you use a ceiling fan (200 watts) for four hours per day, and for 120 days per year, what would be the annual energy consumption?
Use this formula:
Energy Consumption / Day (kWh) = Power Consumption ( Watts / 1000) × Hours Used / Day
Energy Consumption per Day (kWh) = (200 / 1000) × 4 (hours used per day)
Energy Consumption per Day (kWh) = (1/5) × 4
Energy Consumption per Day (kWh) =4/5 or 0.8
So the Energy Consumption per Day is 0.8 kWh To find out energy for 120 days, do simple multiplication: 0.8 x 120 = 96 kWh
Example 2: Calculating Annual Cost of a Ceiling Fan
If the price per kWh for electricity is $0.0845, what is the annual cost to operate the ceiling fan?
Want Another Example?
If you use a personal computer (120 Watts) and monitor (150 Watts) for four hours per day, and for 365 days per year, what would be the annual energy consumption?
So the Energy Consumption per Day is 1.08 kWh. To find out energy for 365 days, do simple multiplication:
The annual cost if electricity is $0.0845 per kWh would be:
Energy Use of Home Appliances, Page 2, Practice
What is the energy consumption of a refrigerator with a wattage rating of 700 Watts when it is operated for 24 hours a day?
Step 1
To solve, use the following formula:
Where:Energy Consumption = Watt Hours (Wh) or KiloWatt Hours (kWh)
Power Consumption = Watts (W) or kW (KiloWatts)
Number of Hours Operated = Hours (h) For the example above:
Energy Consumption = 700 W x 24 h
Energy Consumption = 16800 W h
Step 2
To convert from Wh to kWh, remember that 1kWh = 1000 Wh
To solve, set up as a ratio and use linear algebra to solve for ?.
Practice Problem
Use the following link to generate a random practice problem.
Energy Use of Home Appliances, Page 3
Locating Wattage
You can usually find the wattage of most appliances stamped on the bottom or back of the appliance, or on its "nameplate." The wattage listed is the maximum power drawn by the appliance. Since many appliances have a range of settings (for example, the volume on a radio), the actual amount of power consumed depends on the setting used at any one time.

A refrigerator, although turned "on" all the time, actually cycles on and off at a rate that depends on a number of factors. These factors include how well it is insulated, room temperature, freezer temperature, how often the door is opened, if the coils are clean, if it is defrosted regularly, and the condition of the door seals.
To get an approximate figure for the number of hours that a refrigerator actually operates at its maximum wattage, divide the total time the refrigerator is plugged in by three.
The table below shows wattage of some typical household appliances.
| Appliance | Wattage (range) |
|---|---|
| Clock Radio | 10 |
| Coffee Maker | 900 - 1200 |
| Clothes Washer | 350 - 500 |
| Clothes Dryer | 1800-5000 |
| Dishwasher | 1200-2400 |
| Hair Dryer | 1200-1875 |
| Microwave Oven | 750-1100 |
| Laptop | 50 |
| Refrigerator | 725 |
| 36" Television | 133 |
| Toaster | 800-1400 |
| Water Heater | 4500-5500 |
| Appliance | Wattage |
|---|---|
| Aquarium | 50 - 1210 |
| Clock Radio | 10 |
| Coffee Maker | 900 - 1200 |
| Clothes Washer | 350 - 500 |
| Clothes Dryer | 1800-5000 |
| Dishwasher | 1200 -2400 (using the drying feature greatly increases energy consumption) |
| Dehumidifier | 785 |
| Electric Blanket - Single/Double | 60 / 100 |
| Fan - ceiling | 65 - 175 |
| Fan - window | 55 - 250 |
| Fan - furnace | 750 |
| Fan - whole house | 240 - 750 |
| Hair Dryer | 1200 - 1875 |
| Heater (portable) | 750 - 1500 |
| Clothes Iron | 1000 - 1800 |
| Microwave Oven | 750 - 1100 |
| Personal Computer - CPU - awake / asleep | 120 / 30 or less |
| Personal Computer - Monitor - awake / asleep | 150 / 30 or less |
| Laptop | 50 |
| Radio (stereo) | 70 - 400 |
| Refrigerator (frost free, 16 cubic feet) | 725 |
| 19" Television | 65 - 110 |
| 27" Television | 113 |
| 36" Television | 133 |
| 53" - 61" Projection TV | 170 |
| Flat Screen TV | 120 |
| Toaster | 800-1400 |
| Toaster Oven | 1225 |
| VCR / DVD | 17 - 21 / 20 - 25 |
| Vacuum Cleaner | 1000 - 1440 |
| Water heater (40 gallon) | 4500 - 5500 |
| Water pump (deep well) | 250 - 1100 |
| Water bed (w/heater, no cover) | 120 - 380 |
Amperes and Voltage

is licensed under CC BY-NC-SA 4.0
of the amp meter.
The image shows an ammeter cycling through five different measurements. 118.9 Volts, 7.49 Amps, 885 Watts, 59.9 Hertz, and 0.01 Kilowat Hours.
If the wattage is not listed on the appliance, you can still estimate it by finding the current draw (in amperes) and multiplying that by the voltage used by the appliance.
Most appliances in the United States use 120 volts. Larger appliances, such as clothes dryers and electric cooktops, use 240 volts. The amperes might be stamped on the unit in place of the wattage.
If not, find an ammeter to measure the current flowing through it. You can obtain this type of ammeter in stores that sell electrical and electronic equipment.
Take a reading while the device is running; this is the actual amount of current being used at that instant.
Phantom Loads
Also note that many appliances continue to draw a small amount of power when they are switched "off."
These "phantom loads" occur in most appliances that use electricity, such as VCR, televisions, stereos, computers, and kitchen appliances.
Most phantom loads will increase the appliance's energy consumption a few watts per hour. These loads can be avoided by unplugging the appliance or using a power strip and using the switch on the power strip to cut all power to the appliance.
Review & Extra Resources
Review
Watch this 3 minute 41 second video review Lesson 1
Hello everyone. Dr hall here.
So what we have collected here is a review sheet to help you identify some of the key facts and pieces of information that we covered in the first lesson.
One of the most important things that we covered is that there are six forms of energy and I hope throughout this lesson you will understand each of these and what they represent and you can identify some examples.
Next it's important to note that the forms of energy can be converted from one to another right. So we can go from chemical energy to thermal energy and vice versa depending on the process we're using. Another important factor is that there are some key units that we use to understand energy and quantify and we will switch between a lot of these throughout the course. Some of these are BTUs calories with a capital C, calories with a lowercase c joules kilowatt hours and therms. So each of these are important and quite often used in different fields so you have to be comfortable with each of these because you'll likely see it many times many different circumstances.
So there are also units of power which is distinctly different from energy and that's important to understand and appreciate the difference. The units for power are watts, kilowatts, joules per second, horsepower, and calories per second.
We also touched on the sources of energy some of them are renewable and here we define renewables those that can be replenished over and over again and are not depleted. Some examples of those are hydropower solar wind tidal and geothermal energy. There are also non-renewable sources and the way we define these is that they cannot be replenished over and over again in a reasonable amount of time. Some of these include fossil fuels tar sands and nuclear fission. So they rely on finite sources that take a long time to form. And as a part of our sources of energy section we also covered fossil fuel distribution uh across the world and in the us so that you can understand where these finite resources are and how much we have.
So in covering all of these previous topics you'll also note that there are a few important definitions. Power is the rate at which we do work right so it's how much work we can get done in a given amount of time. Energy is our capacity to do work and work is the amount of things that we get done. And many of these are interrelatable so for example power is energy divided by some period of time. Energy is power multiplied by a duration of usage. And energy consumption per day is equal to power consumption times those hours used within a day.
So i think if you focus on each of these key concepts here and the fact sheet provided to you I think you'll find them easier and there will also be some practice questions in which these concepts will help okay. So please study this practice the practice questions frequently frequently and when you're ready please proceed to the quiz.
Okay, good luck everyone.
Review Sheet - Energy and Society
- Forms of Energy
- Mechanical energy
- Potential Energy
- Kinetic Energy
- Chemical Energy
- Thermal or Heat Energy
- Electrical Energy
- Nuclear Energy
- Fission
- Fusion
- Radiation
- Mechanical energy
- Electromagnetic Spectrum
- The lower the energy, the longer the wavelength and lower the frequency, and vice versa
- Energy can be converted from one form to another
- Units of Energy
- BTU, Calorie, calorie, Joules, kWh, Therm
- Food Calorie (usually written with 'C')
- calorie (usually written with 'c')
- 1 Food Calorie = 1000 calories
- Units of Power
- Watts
- kW (kilo-watts)
- J/s
- HP
- cal/s
- Sources of Energy
- Renewable
- Can be replenished over and over again; they are never depleted
- Hydropower, Solar, Wind, Tidal, Geothermal energy from inside the earth, Biomass from plants, Nuclear Fusion
- Non-renewable
- Cannot be replenished over and over again; they get depleted
- Fossil fuels, Tar Sands, Nuclear Fission
- Fossil Fuel Distribution
- US has a lot of Coal reserves
- Middle East has a lot of petroleum reserves
- Renewable
- Definitions
- Power is the rate at which we do work
- Energy is the capacity to do work
- Work is the amount done
- Power = Energy / Time
- Energy = Power x Duration of Usage (time)
- Energy consumption/day = Power consumption x hrs used/day
Extra Resources
For more information on topics discussed in Lesson 1, see these selected references:
Lesson 1 Deliverable
Deliverable
You must complete a short quiz that covers the reading material in lesson 1. The Lesson 1 Quiz can be found in the Lesson 1: Energy and Society module in Canvas. Please refer to the Calendar in Canvas for specific time frames and due dates.
Lesson 2: Energy Supply and Demand
The links below provide an outline of the material for this lesson. Be sure to carefully read through the entire lesson before returning to Canvas to submit your assignments.
Introduction
Welcome to Lesson 2!
You are already to Lesson 2: Energy Supply and Demand. These energy supply and demand lessons will explore more about energy resources. Basically, you will be able to appreciate global and national consumption patterns. We will go over what kinds of energy we have been consuming, how much we have been consuming, and how we compare with the rest of the world, etc., over the past few decades. Based on those patterns, we can also deduce some information about how much energy we use to do a job; energy intensity. What kind of energy sources we will be needing, and how much we will be needing based on the past trends?
We will also look at energy reserves. Do we have those? Will we need more renewables, batteries, coal, more oil, or more gas? Can we (sustainably) use any of those resources? If we can, great, if not, what do we do? We will be doing those kinds of energy analysis. So, obviously, you are going to see a lot of numbers and statistics. One of the questions that I always get is: Hey, do I have to remember all these numbers? In 2019, petroleum and natural gas accounted for 69 % of energy consumption in this country, and renewables accounted for 11% of the electricity generated by utility companies.
Do we have to remember these numbers? My advice is, you don’t have to remember everything but you need to get the main message behind these numbers. In other words, you have to know the fact that petroleum and natural gas are the main energy sources. Similarly, over 75% of our oil products or petroleum products are used for transportation. So, we need to know that transportation is basically run by petroleum; petroleum is mostly used for transportation. That is the message. You don’t need to remember the exact numbers. But if there is something that has very insignificant or significant quantities, you should note that. For example, renewable energy sources supply about 10% of our energy source (although the exact number is about 11%). That is the message. You don’t need to worry about whether it is 9.2 or 9.5 or 8.5 or 11.2%. So just to give you a clue, you don’t have to worry about the exact numbers, but the message that is conveyed using these numbers is what you have to concentrate on. And we will also have one numerical type of problem in this lesson. That problem will be predicting the energy needed for the future. The problem follows an exponential function, and we will talk about that, and there will be a few numerical problems for you to practice.
See the Calendar tab in Canvas for due dates/times.
Questions?
If you have any questions, please post them to the General Course Questions forum in located in the Discussions tab in Canvas. I will check that discussion forum daily to respond. While you are visiting the discussion board, feel free to post your own responses to questions posted by others - this way, you might help a classmate!
Global Energy Consumption
Energy consumption numbers are always reported a few years behind, so we are always looking into the past before we plan for the future. As of 2021, The world's total primary energy consumption was about 176,000 TWhs (580 Quadrillion Btus).
Table 2.1 below shows various regions and total primary energy consumption history.
| Region | 1980 | 1985 | 1990 | 1995 | 2000 | 2005 | 2010 | 2015 |
|---|---|---|---|---|---|---|---|---|
| North America | 91.6 | 91.0 | 100 | 109 | 118 | 121 | 118 | 119.9 |
| Central and S. America | 11.5 | 12.3 | 14.5 | 17.6 | 20.8 | 23.2 | 26.9 | 29.7 |
| Europe | 71.8 | 72.9 | 76.3 | 76.7 | 81.5 | 85.8 | 83.8 | 81.2 |
| Eurasia | 46.7 | 55.7 | 61.0 | 42.2 | 39.2 | 43 | 42.8 | 44.8 |
| Africa | 6.8 | 8.5 | 9.5 | 10.7 | 12.0 | 14.5 | 16.3 | 19.3 |
| Asia & Oceania | 48.9 | 58.1 | 73.4 | 93.5 | 111 | 149 | 194 | 239.9 |
| WORLD TOTAL | 283 | 307 | 346 | 363 | 400 | 459 | 511 | 570.4 |
From the Table 2.1, it can be seen that the energy consumption is high for the Far East and Oceania. The amount of energy used depends on the economic prosperity of the nation and the population of the country. The North American region includes Canada, the United States, and Mexico. The Far East and Oceania include developed nations such as Japan and Australia, and densely populated developing nations such as China and India. Obviously, due to highly populated countries like China and India, total energy consumption does not reflect the quality of life of the people in those countries. Figure 2.1 shows the energy per capita consumption over the last 30 years. As can be seen from the graph that Far East and Oceania used a lot of energy as a region, but per capita energy consumption is relatively low implying that if each person in that region would consume as much as in North America, that Region's energy consumption would skyrocket.

The productivity of a country is measured by the total value (dollars) of goods and services, called Gross Domestic Product (GDP), produced by its people. Therefore, the average value of goods and services produced by each person - the GDP per capita of a country - is an indicator of the quality of life.
Energy intensity is the relationship between energy consumption and growth in gross domestic product (GDP), and it is an important factor that affects changes in energy consumption over time.
- In industrialized countries, history shows the link between energy consumption and economic growth to be a relatively weak one, with growth in energy demand lagging behind economic growth.
- In developing countries, however, the link between energy consumption and economic growth have been more closely correlated with energy demand growing in parallel with economic expansion.
The total primary energy consumption of the world in 2015 was 570 Quadrillion Btus and in 2017 it increased to 582.4


Global Energy Consumption and GDP per person
In general, as the GDP (Gross Domestic Product) per person of any country increases, the amount of energy that is consumed is also expected to increase.
- For developing nations, the correlation is much stronger.
- For developed nations, the correlation is weak.
For example, Iceland, Finland, the United States, and the Netherlands, with similar GDP per capita, have significant differences in energy consumption per capita. In other words, to produce one dollar's worth of goods and services, the U.S. uses twice the energy of the Netherlands. Similarly, Iceland uses four times the energy of the Netherlands.

Energy Consumption Differences
The differences in energy consumption among countries are the result of:
- efficiency of industrial, transportation, commercial, and residential energy,
- climatic and geographical areas of a country,
- lifestyles (use of more gas guzzling cars and SUVs and bigger sized houses), and
- the nature of the products produced by the nation's industries.

World Energy Outlook
The world energy requirements are projected by several energy companies such as British Petroleum and Exxon Mobil and international agencies like International Energy Agency (IEA) and US Energy Information Administration (EIA). The United States Department of Energy projects strong growth for worldwide energy demand over the 28-year projection period from 2012 to 2040. Although these projections or forecasts are based on the same principles, they differ slightly. They are also often wrong! For example, the rate of renewable adoption is continuously underestimated. Detailed projections of each organization can be seen through the following external links.
- International Energy Agency
- British Petroleum
- Exxon Mobil
- US Energy Information Administration (EIA)
According to International Energy Outlook 2019,
- Manufacturing centers are shifting toward Africa and South Asia, especially India, resulting in energy consumption growth. Most economic growth happens in non-OECD countries, where GDP per person nearly triples from 2018 to 2050.
- The total world energy consumption is likely to increase from 591 quadrillion Btus in 2016 to 910 quadrillion Btus in 2040, an increase of 54%.
- Most economic growth happens in non-OECD countries, where GDP per person nearly triples from 2018 to 2050. Most energy-intensive manufacturing shifts to non-OECD Asia and, increasingly, to India.
The world's GDP (expressed in purchasing power parity terms) rises by 2.4%/year from 2018 to 2040. The fastest rates of growth are projected for the emerging, non-OECD countries, where combined GDP increases by 3.5%/year. In OECD countries, GDP grows at a much slower rate of 1.5%/year over the projection period.
 Asia is heavily populated and continues to grow at a rapid pace. As a result, industrial growth has also increased, requiring a need for more energy.
Asia is heavily populated and continues to grow at a rapid pace. As a result, industrial growth has also increased, requiring a need for more energy.
Current and Future Energy Sources of the World
The World’s energy supply sources
Before the global pandemic, the World’s energy supply sources followed long-term trends, with new fuels increasing in share and old fuels losing ground. Below, you can see consumption history for the years 1990 to 2018.

Here we can see the role of renewables and natural gas quickly growing.
Three of the world’s largest energy sources
| Source | Future Outlook | Advantages / Disadvantages |
|---|---|---|
| Oil | Over the past four decades, oil has been the world's foremost source of primary energy consumption, and it is expected to remain in that position throughout the projected time frame. Liquids (primarily oil and other petroleum products) are expected to continue to provide the largest share of world's energy consumption over the projected period. | In the transportation sector, in particular, liquid fuels continue to provide most of the energy consumed. Although advances in nonliquids-based transportation technologies are anticipated, they are not enough to offset the rising demand for transportation services worldwide. As a result, oil is projected to retain its predominance in the global energy mix and meet 30% of the total primary energy consumption in 2040. |
| Natural Gas | Worldwide natural gas consumption is projected to increase from 120 trillion cubic feet (Tcf) in 2012 to 203 Tcf in 2040. By energy source, natural gas accounts for the largest increase in world primary energy consumption. Abundant natural gas resources from shale resources and robust production contribute to the strong competitive position of natural gas among other resources. Natural gas remains a key fuel in the electric power sector and in the industrial sector. | It is seen as the desired option for electric power, given its relative efficiency and environmental advantages in comparison with other fossil energy sources.
Natural gas burns more cleanly than either coal or oil, making it a more attractive choice for countries seeking to reduce greenhouse gas emissions. |
| Coal | Coal is the world’s slowest-growing energy source, rising by an average 0.6%/year, from 153 quadrillion Btu in 2012 to 180 quadrillion Btu in 2040. Throughout the projection, the top three coal-consuming countries are China, the United States, and India, which together account for more than 70% of world coal use.
Coal use will continue to increase in developing countries, but in developed or industrialized countries, it will not increase but may slightly decrease. |
Global coal production is projected to increase from 9 billion short tons in 2012 to 10 billion short tons in 2040. Most of the projected growth in world coal production occurs in India, China, and Australia.
Coal remains a vital fuel for world’s electricity markets and is expected to continue to dominate energy markets in developing Asia. |
Energy Consumption and Electricity Projections
According to International Energy Outlook 2019, the strongest growth in electricity generation is projected to occur among the developing, non-OECD nations. Increases in non-OECD electricity generation average 2.5%/year from 2012 to 2040, as rising living standards increase demand for home appliances and electronic devices, as well as for commercial services, including hospitals, schools, office buildings, and shopping malls. In the OECD nations, where infrastructures are more mature and population growth is relatively slow or declining, electric power generation increases by an average of 1.2%/year from 2012 to 2040.

US Energy Information Administration, International Energy Agency. 2010.

US Energy Information Administration, International Energy Agency. 2010.
As can be seen from the figures, the role of renewables is expected to change dramatically in the coming years. What a transition!
Nuclear Power
Worldwide, electricity generation from nuclear power is projected to remain largely unchanged into 2040.
According to International Energy Outlook projections by US Department of Energy (US DOE), there is still considerable uncertainty about the future of nuclear power, and a number of issues could slow the development of new nuclear power plants. Issues related to plant safety, radioactive waste disposal, and proliferation of nuclear materials continue to raise public concerns in many countries and may hinder plans for new installations. Although the long-term implications of the disaster at Japan's Fukushima Daiichi nuclear power plant for world nuclear power development are unknown, Germany, Switzerland, and Italy have already announced plans to phase out or cancel all their existing and future reactors. In contrast, developing Asia is poised for a robust expansion of nuclear generation. Most of the increase is by China's addition of 139 gigawatts (GW) of nuclear capacity from 2012 to 2040.

In a nuclear plant, heat is produced by nuclear fission (splitting of an atom's nucleus into many new atoms) inside uranium fuel. As a result of fission, heat energy is released and the steam spins a turbine generator to produce electricity.
Renewables
Sizable growth in the world’s consumption of renewable energy resources is projected over the next 25 years. Much of the projected growth in renewable generation is expected to result from the completion of solar installations all throughout the world. Dramatic reductions in the cost of solar panels has led to this source being one of the cheapest forms of electric power generation as of 2020. In 2020, 90 % of all new electric power generation installations were renewable across the globe.

In hydroelectricity, mechanical energy from the water being pulled downward by gravity is converted to electrical energy. More specifically, a hydroelectric generator directs the flow of water through a turbine, which extracts the kinetic energy from the movement of the water and turns it into electricity through the rotation of electrical generators. Hydropower is the largest single renewable electricity source today, providing 16% of world electricity at competitive prices. It dominates the electricity mix in several countries, developed, emerging, or developing. However, wind and solar are quickly catching up.
Growth in Energy Demand
For a long time, growth in the world and the U. S. energy consumption as a function of time, follow what is known as exponential function. Now it looks like we have switched to linear growth, but time will tell if this is a permanent change. The exponential increase is characterized as follows. The amount of change (increase in energy consumption) per unit time is proportional to the quantity (or consumption) at that time.
or
Where Greek letter Δ(delta) is the change or increment of the variable and λ (lambda) is the growth rate. After some mathematical methods, it can be shown that the equation changes to the form
where e is a constant = 2.71
We can determine how long it takes for N0 to become 2N0 (twice its original number or double). That time period is called doubling time. After some mathematical steps it can be written as:
Illustration
If the use of energy is projected to increase at the rate of 1.7% per year in the U.S. How long will it take to double its usage?
In 41.17 years, the consumption of energy will be twice as much as it is today.Energy Reserves
Until so far that the energy requirement is going to increase in the future and also that the U.S. and the rest of the world will depend to some extent on fossil fuels. These fossil fuels are non-renewable fuels with a finite lifetime. So, the question is: Will we have enough supply for future energy requirements?
The answer to this question depends on the quantity of fossil fuels we have in the ground. Energy sources that have been discovered but not produced cannot be easily measured. Trapped several feet below the surface, they cannot be measured with precision. There are several terms used to report the estimates of the energy resources. The most commonly used terms are “reserves” and “resources.”
- "Reserves" represent that portion of demonstrated resources that can be recovered economically with the application of extraction technology available currently or in the foreseeable future. Reserves include only recoverable energy.
- “Resources” represent that portion of the energy that is known to exist or even suspected to exist, irrespective of technical or economic viability. So reserves are a subset of resources.
| Source of Energy | U.S. Reserves | U.S. Annual Consumption | World Reserves | World Annual Consumption |
|---|---|---|---|---|
| Petroleum (billions of barrels) |
35 | 7.21 | 1707 | 35 |
| Natural gas (Wet) Trillion Cu. Ft. |
324 | 32.1 | 6588 | 124 |
| Coal (billions of short tons) |
260 | 0.73 | 948 | 8.28 |
As of 2016, total world proved recoverable reserves of coal were estimated at 948 billion short tons.
Five countries have nearly 73% of the world's coal reserves:
- United States—28%
- Russia—18%
- China—13%
- Australia—9%
- India—7%
Based on data from OPEC (Oil Producing and Exporting Countries), the highest proved oil reserves including non-conventional oil deposits are shown in the graphic below.
Based on data from BP (British Petroleum), proved gas reserves were dominated by three countries: Iran, Russia, and Qatar, which together held nearly half the world's proven reserves. According to the US CIA The World Factbook, the US has the 5th largest reserves of natural gas. Due to constant updates about the shale gas estimates, these are difficult to say with certainty.
How Long Will the Reserves Last?
How long these reserves do last depends on the rate at which we consume these reserves. For example, let’s assume that we have $100,000 in the bank (reserves) and if we draw 10,000 dollars every year (consumption) the reserve will last for 10 years (\$100,000/\$10,000 per year). However, in this case, we are assuming that we do not add any money to our deposit, and we do not increase our withdrawal.
This is generally not true in the case of life of an energy reserve. We may find new reserves, and our energy consumption or production can also increase. In the case of energy reserve, although we know that we might find new resources, we do not know how much we could find. But the consumption can be predicted with some accuracy based on the past rates.
Lifetime of current reserves at constant consumption
We can calculate the life of current petroleum reserves by dividing the current reserves by current consumption.
- At the current rate of consumption, the approximate lifetime of the world’s petroleum, natural gas, and coal reserves is 50 years, 52.8 years, and 153 years, respectively. (BP Statistical Review of World Energy)
- At the current rate of consumption, the current U. S. petroleum, natural gas, and coal reserves will last approximately for 4.88 years, 12.2 years, and 258 years, respectively.
It is important to note that the entire U.S. petroleum consumption is not coming from the U.S. reserves because we import more than one half of the consumption. Because we import more than one half of the consumption, the petroleum reserves at the current rate will last about 11 years. If the consumption increases in the future, the life will be less. However, there is also a chance of adding more reserves with more exploration and discoveries. The increase in consumption can change depending on the price of petroleum and other alternative fuels. Likewise, us moving to electric cars and harnessing unconventional oil reserves can extend the lifetime of these reserves.
Therefore, these lifetimes are not carved in stone. It can be debated whether the U.S. reserves will last for 6 years or 10 years or even 20 years, or we may never run out! But there is increasing consensus that we must change our lifestyle. Even if we won't run out, the environmental consequences of continued use are pushing us to change anyway, but more on that later..
The R/P ratio can change from year to year, similar to our bank balance. We can add more if we make more or consume more. That changes the time we can draw on the balance.
The following figures illustrate that these ratios changed.

Therefore, we must conserve, innovate (get more with less), or learn to live without these resources.
Current and Future Energy Sources of the USA
US Energy Supply and Demand
When focusing on the energy supply and demand, it can be helpful to see where the energy is going. Which of our daily activities consume the most energy? Several agencies track this very closely.

Globally, many interesting transitions are occurring. While the U.S. was once the top consumer of energy, China claimed this title in 2009.
| Country | Consumption in Exajoules |
|---|---|
| China | 141.70 |
| U.S. | 94.65 |
| India | 34.06 |
| Russia | 29.81 |
As a result of innovations in oil and gas extraction, U.S. imports have dropped considerably.
Looking at the U.S. Energy Profile, It can be seen from the imports profile that the US Crude oil imports have significantly reduced between 2005 and 2019 from a peak of 25 Quadrillion BTUs. Another significant change that can be noted is that the US is now exporting natural gas (below zero on the y axis) instead of importing it. Although crude oil is imported, US exports finished petroleum products resulting in less net imports. As a matter of fact, US total energy exports exceeded the imports in 2019 since 1950.
The top five countries (sources) of US total petroleum in 2019 were Canada (49%), Mexico (7%), Saudi Arabia (6%), Russia (6%) and Columbia (4%).
The U.S. also ranks:
- first in worldwide reserves of coal;
- sixth in worldwide reserves of natural gas;
- eleventh in worldwide reserves of oil.
US Energy Consumption by Source and the chart of the US Energy consumption by source and user sector shows each energy source and the amount of energy it supplies in British thermal units (BTU). Petroleum is the leading source of energy in the US in 2019 with 36.72 quadrillion BTUs. Next is natural gas with 32.10 quadrillion BTUs. Coal supplies 11.31 quadrillion BTUs of energy. Renewable energy and nuclear power are responsible for 11.46 and 8.46 quadrillion BTUs respectively. Of the total petroleum consumption, 72% is used for transportation and another 23% is used by the industrial sector. Similarly, 35% of the natural gas (largest fraction) is used for power generation. On the other hand, 76% of the residential and commercial energy needs are met by natural gas. The actual percentages are not required to be memorized but answers to the questions such as: Which fuel is most used by power plants for power generation? Which sector uses petroleum the most? Approximately what fraction of the electricity is generated by renewable energy? (10, 25, 50 or 90) What is the primary purpose of coal use? etc. need to be answered.
US Energy Consumption by Source and Sector
The graph shows how dependent the U.S. is on our petroleum supply, as it accounts for almost 37% of our energy. Our next two highest sources of energy, like petroleum, are non-renewable and include natural gas and coal. Only about 11% of our energy comes from renewable energy sources such as wood and water (hydroelectricity). According to Energy Information Administration, US renewable energy consumption surpassed coal for the first time in over 130 years in 2019. Of the 4.12 trillion kWh of electricity generated in the US, 38% was from natural gas, coal accounted for about 23% and nuclear adding another 20%. Renewable sources contributed to 17% of the total electricity generated.
In 2019, fossil fuels made up 81% of total U.S. energy consumption, the lowest fossil fuel share in the past century. According to EIA projections, the percentage declines to 76.6% by 2040. Policy changes or technology breakthroughs that go beyond the trend improvements could significantly change that projection. In 2019, the renewable share of energy consumption in the United States was its largest since the 1930s at nearly 11.7%. The greatest growth in renewables over the past decade has been in solar and wind electricity generation. Liquid biofuels have also increased in recent years, contributing to the growing renewable share of total energy consumption. 2020 was the first year that renewables surpassed coal consumption in the U.S.
US Energy Consumption Over Time

The most significant decline in recent years has been coal: U.S. coal consumption fell by 41% since 2009 in a decade, from 997 million tons to 587 million tons. Biomass, which includes wood as well as liquid biofuels like ethanol and biodiesel, remain relatively flat, as wood use declines and biofuel use increases slightly. In contrast, wind and solar are among the fastest-growing energy sources in the projection, ultimately surpassing biomass and nuclear.
Net U.S. imports of energy declined from 30% of total energy consumption in 2005 to 13% in 2013 and down to 7.6 % in 2017, as a result of strong growth in domestic oil and dry natural gas production from tight formations and slow growth of total energy consumption.
US Energy Consumption History
The plot of US energy consumption shows the relative amounts of each type of energy that was consumed for each year. The history of the energy consumption profile of the United States indicates that petroleum makes the largest part of the energy demand over the past seven decades. Natural gas has taken the second over the past decade with the production of gas from shale. Coal has been replaced by renewable energy and natural gas for electricity generation. Among the renewable energy sources, biomass has the larger share followed by wind energy. Wind energy and solar energy are the fastest growing energy sources.

Answers to the following questions need to be looked for in the material presented above.
- Of all the renewable energy sources, which renewable source is used most?
- Which of the renewable sources is used most for transportation?
- Approximately what fraction of the electricity is generated by nuclear energy? (10, 20, 50 or 90)
| Source | Future Outlook | Advantages / Disadvantages |
|---|---|---|
| Oil | Petroleum demand is projected to grow from 19.17 million barrels per day in 2010 to 19.9 million barrels per day in 2035. | Approximately 72.1% of the petroleum in the U. S. is used for transportation, and about 22.5% is used by the industrial sector. |
| Natural Gas |
Total dry natural gas production in the United States increased by 35% from 2005 to 2013, with the natural gas share of total U.S. energy consumption rising from 23% to 28%. Production growth resulted largely from the development of shale gas resources. In 2009, production stood at 20.65 trillion cubic feet (Tcf), net imports at 2.68 Tcf, and consumption at 22.85 Tcf. The projections for domestic natural gas consumption in 2030 is 26.1 trillion cubic feet per year, as compared with 24.13 trillion cubic feet in 2010. In the reference case, natural gas consumption in the electric power sector is projected to increase from 7.38 trillion cubic feet in 2010 to a peak of 8.08 trillion cubic feet in 2015. Natural gas use in the electric power sector levels off after 2020. Continued growth in residential, commercial, and industrial consumption of natural gas is roughly offset by the projected decline in natural gas demand for electricity generation. As a result, overall natural gas consumption is almost flat between 2020 and 2025. |
Energy Information Administration forecasts greater dependence on more costly supplies of natural gas, such as imports of Liquefied Natural gas (LNG), and remote resources from Alaska and the Mackenzie Delta in Canada. LNG imports, Alaskan production, and production in the 48 States from nonconventional sources are not expected to increase enough to offset the impacts of resource depletion and increased demand. The industrial sector was the largest consuming sector of natural gas. Production of gas from shale (such as Marcelleus) is likely to change the natural gas usage very quickly. Gas can be converted to oil. New gas to liquid fuel plants in the US are likely to change the oil imports scenario in the next two decades. |
| Coal |
Between 2008 and 2013, U.S. coal production fell by 187 million short tons (16%), as declining natural gas prices made coal less competitive as a fuel for generating electricity U.S. coal production increases at an average rate of 0.7%/year from 2013 to 2030, from 985 million short tons (19.9 quadrillion Btu) to 1,118 million short tons (22.4 quadrillion Btu). Over the same period, rising natural gas prices, particularly after 2017, contribute to increases in electricity generation from existing coal-fired power plants as coal prices increase more slowly. Renewables and additional natural gas plants continue to replace coal driving down use. |
Compliance with the Mercury and Air Toxics Standards (MATS), coupled with low natural gas prices and competition from renewables, leads to the projected retirement of 31 gigawatts (GW) of coal-fired generating capacity and the conversion of 4 GW of coal-fired generating capacity to natural gas between 2014 and 2016. However, coal consumption in the U.S. electric power sector is supported by an increase in output from the remaining coal-fired power plants, with the projected capacity factor for the U.S. coal fleet increasing from 60% in 2013 to 67% in 2016. In the absence of any significant additions of coal-fired electricity generating capacity, coal production after 2030 levels off as many existing coal-fired generating units reach maximum capacity factors and coal exports grow slowly. Total U.S. coal production remains below its 2008 level through 2040. |
US Vehicle Fuel Consumption
The table below shows the vehicle fuel consumption and travel between 1960 and 2018.
| - | 1960 | 1970 | 1980 | 1990 | 2000 | 2010 | 2018 |
|---|---|---|---|---|---|---|---|
| Vehicles registered (thousands)a | 73,858 | 111,242 | 161,490 | 193,057 | 225,821 | 250,070 | 273,602 |
| Vehicle-miles traveled (millions) | 718,762 | 1,109,724 | 1,527,295 | 2,144,362 | R2,746,925 | 2,967,266 | 3,269,088 |
| Fuel consumed (million gallons) | 57,880 | 92,329 | 114,960 | 130,755 | R162,554 | 169,679 | 176,444 (2016) |
| Average miles traveled per vehicle (thousands) | 9.7 | 10.0 | 9.5 | 11.1 | 12.2 | 11.8 | 11.9 |
| Average miles traveled per gallon | 12.4 | 12.0 | 14.9 | 18.8 | 20.0 | 21.5 | 22.3 |
| Average fuel consumed per vehicle (gallons) | 784 | 830 | 712 | 677 | R720 | 678 | 644 |
Key: R = revised a Includes personal passenger vehicles, buses, and trucks. Source: 1960-94: U.S. Department of Transportation, Federal Highway Administration, Highway Statistics Summary to 1995, FHWA-PL-97-009 (Washington, DC: July 1997), table VM-201A.
1995-2005: Ibid., Highway Statistics (Washington, DC: Annual issues), table VM-1
Source: U.S. Department of Transportation, Federal Highway Administration, Highway Statistics 2010
Electricity
US electricity generation is expected to grow from 3.7 trillion kilowatthours in 2015 to 4558 trillion kilowatthours in 2035. Visit the webpage US electricity explained - Sources and profiles
Examine for
- Sources of U,.S electricity generation
- What are the notable changes in the major sources for electricity generation between 1950 and 2019?
- Role of renewable energy in the electricity generation.
Growth in electricity use for computers, office equipment, and electrical appliances in the residential and commercial sectors is partially offset by improved efficiency in these and other, more traditional electrical applications, by the effects of demand-side management programs, and by slower growth in electricity demand for some applications, such as air conditioning.
Most capacity additions over the next 10 years are renewables and natural gas, increasing the natural gas share to 26 percent and lowering the coal share to under 20 percent. The nuclear generation (about 9 percent of total electricity supply in 2012) is projected to remain at about 9 percent in 2050.
Did You Know?
Demand-side management programs address efficiency. By being more efficient, we can do more with less, and then reduce the demand for energy.
Review & Extra Resources
Review
Watch the following 4 minute 50 second video Review for Lesson 2.
Hello again, Dr. hall here.
So you've finished lesson. two on energy supply and demand and again we've made a review sheet to help you identify some of the key components of this lesson to better prepare yourself for the quiz.
So one of the first points is gross domestic product. You may have seen this in an economics class but we're introducing this so you understand the relationship between productivity and nations and how that relates to energy use.
Another important concept is energy intensity so you can get a sense for how much each citizen in the world uses energy and how that varies across the globe. So because we're talking about national and global energy consumption and production, the units of energy that we're using are extraordinarily large. So we talk about quads or quadrillion BTUs which is 10 to the power 15 BTUs. That's right, so that's a lot of energy. A tremendous amount compared to how much we need to power our bodies in the day.
Right so the given amount of calories we consume or about 2,000 and comparing that to how many btus we use to do everything else in our life it's quite extraordinary what the differences are.
So we also covered world energy consumption and some of the trends that we've seen in the past and what we expect again the best of our knowledge what we expect they will look like in the future.
And there are some key points that you can learn from this. So number one oil is one of the most utilized energy resources across the world and it seems like it'll stay that way from 2020 on to 2050. Another fact that we're coming to terms with is energy consumption across the globe will likely continue to increase from 2020 on to 2050. In terms of the u.s, we have an extraordinary amount of fossil fuels in particular coal. But like much of the rest of the world what we use regularly the most is still oil and it looks like it will remain that way for a long time in the future.
Another thing that seems to be a constant is that the consumption of all different energy sources both renewable and non-renewable appear today will increase to meet our growing energy demand. Uh as you'd expect or what you you'd probably be familiar with in your real life. most of our use of petroleum and oil is in the form of transportation and that's why it's so high is we we move many things in this world and a lot of that relies on petroleum-based products. And in the U.S. we use so much of this that we regularly have to import this petroleum from somewhere else because we don't produce as much as we would need in order to satisfy the demand. And lastly the U.S. not only has a lot of coal in terms of itself but even in terms of the world we have about one fourth of the resorts overall.
Another interesting thing to consider is the doubling time ,right. This was important concept that we covered that helps us understand the role of growth and consumption how that relates to uh the supply of energy and the consumption that we have of it.
Uh when we talk about energy in terms of non-renewable sources. We have what's called reserves and resources and each of these represent two distinctly different qualities that we have in an energy resource. So when we look at those we can also do some rather straightforward math to see how long we expect those reserves to last. But in an ever and changing world where our relationship with energy keeps changing we can always estimate how long those reserves last but innovations sometimes improve how much we we have in terms of resources and reserves and it also changes our relationship with how we use them. Right so for example the emergence of renewable energy and electric cars that changes how long those reserves are last because it changes our our rate of consuming them.
So uh again uh i'm glad that you finished lesson two. I hope that you take into account each of these points uh review. Be sure to practice the practice questions and good luck on the quiz alright.
Thanks everyone.
Review Sheet – Energy Supply and Demand
- Gross Domestic Product (GDP)
- Energy Intensity
- Quadrillion Btus = 1015 Btus
- World Energy Consumption
- Oil is the most utilized energy source in both 2020 and 2050
- Energy Consumption will increase from 2020 to 2050
- United States Energy Consumption
- First in worldwide reserves of coal
- Oil is the most utilized energy source in both 2020 up to 2050.
- Consumption of all energy sources will increase from 2020 to 2050
- 66.5% of petroleum is used for transportation
- More than half of petroleum needs are met by imports
- US has almost one fourth of the world’s reserves of coal
- Doubling time
- Energy reserves and resources
- "Reserves" represent that portion of demonstrated resources that can be recovered economically with the application of extraction technology available currently or in the foreseeable future. Reserves include only recoverable energy.
- “Resources” represent that portion of the energy that is known to exist or even suspected to exist irrespective of technical or economic viability. So reserves are a subset of resources.
- How long will the reserves last?
Test Yourself
The questions below are your chance to test and practice your understanding of the content covered in this lesson. In other words, you should be able to answer the following questions if you know the material that was just covered! If you have problems with any of the items, feel free to post your question on the unit message board so your classmates, and/or your instructor, can help you out!
- Why is the energy use per person in the world increasing?
- The United States, with 5% of the world's population, uses about 25% of the world's energy and contributes 25% of the world's greenhouse gas emissions. Explain.
- List the reasons why the United States per capita energy consumption is the highest of any other region in the world.
- List reasons why the United States energy consumption per dollar of GDP is higher than most of the industrialized nations.
- What is the difference between reserves and resources?
- List the changes that you would make in your personal lifestyle if you were mandated to reduce your energy consumption by 25%.
- What variables determine the lifetime of a nonrenewable resource?
Extra Resources
For more information on topics discussed in Lesson 2, see these selected references:
- Hinrichs, R. A., “Energy,” Saunders College Publishers, Philadelphia, PA, 1992.
- Aubrecht, G. L., “Energy,” Prentice Hall, Inc., Englewood Cliffs, NJ, 1995.
- Fay, J.A. and Golomb, D. S., “Energy and the Environment,” Oxford University Press, New York, NY, 2002.
- Christensen, J. W., “Global Science: Energy Resources Environment”, 4th edition, Kendall/Hunt Publishing Company, Dubuque, IA, 1996.
Energy Information Administration, Annual Energy Review, U.S. Department of Energy, 2004. - Energy Information Administration, Annual Energy Outlook, DOE/EIA 0383 (2004), U.S. Department of Energy, Washington D.C., 2004.
- Energy Information Administration, International Energy Outlook, DOE/EIA 0484 (2004), U.S. Department of Energy, Washington D.C., 2004.
Lesson 2 Deliverable
Deliverable
You must complete a short quiz that covers the reading material in lesson 2. The Lesson 2 Quiz can be found in the Lesson 2: Energy Supply and Demand module in Canvas. Please refer to the Calendar in Canvas for specific time frames and due dates.
Lesson 3: Energy Efficiency
The links below provide an outline of the material for this lesson. Be sure to carefully read through the entire lesson before returning to Canvas to submit your assignments.
Introduction
Welcome to Lesson 3!
Alright, we are into lesson 3. Lesson 3 basically deals with energy efficiency. Upon completing this lesson, you will really get a quantitative feel for what energy efficiency is and also how to calculate energy efficiency with a conversion device. A conversion device can be anything – an automobile or a power plant or any device that converts one form of energy to another form.
And we will also understand the concept of entropy. Entropy is disorder. Because of entropy, our efficiencies are lower than what we normally expect. And we will look at the operating principle of a heat engine. A heat engine is a device that converts heat to work. Particularly, automobiles are all heat engines, and they are notoriously inefficient. We will see 2 examples and calculations of why these automobiles are notoriously inefficient.
We can also calculate the efficiency of a whole process from the step efficiencies. For example, if it involves 2 or 3 steps like in a relay race. You know you have 3 or 4 players taking the baton and one lap by each of the athletes. So, what is the overall or team efficiency if we know the efficiency of each of those steps or the efficiency of each of those players? That is a very important concept in this chapter.
While doing this, we will also learn about temperature scales; Kelvin scale, Fahrenheit, and also Celsius. So there will be a lot of numerical problems in this lesson.
Alright! Good luck!
Lesson 3 Objectives
Upon completing this lesson, you should be able to:
- define and calculate efficiency of an energy conversion device;
- articulate the concept of entropy;
- explain operating principles of a heat engine; and
- calculate overall efficiency from step efficiencies.
See the calendar in Canvas for due dates/times.
Questions?
If you have any questions, please post them to the General Course Questions forum in located in the Discussions tab in Canvas. I will check that discussion forum daily to respond. While you are visiting the discussion board, feel free to post your own responses to questions posted by others - this way, you might help a classmate!
Energy Conversion Devices
In the first lesson, we saw that energy can be transformed from one form to another, and during this conversion, all the energy that we put into a device comes out. However, all the energy that we put in may not come out in the desired form.
For example, we put electrical energy into a bulb and the bulb produces light (which is the desired form of output from a bulb), but we also get heat from the bulb (undesired form of energy from an electric bulb).

Therefore, energy flow into and out of any energy conversion device can be summarized in the diagram below:

When all forms of energy coming out of an energy conversion device are added up, it will be equal to the energy that is put into a device. Energy output must be equal to the input. This means that energy can not be destroyed or created. It can only change its form.
In the case of an electric bulb, the electrical energy is converted to light and heat.
The amount of electrical energy put into a bulb = the amount of light energy (desirable form) plus the heat energy that comes out of the bulb (undesirable form).
 Say you go to the mall with $100, and you come back with only $10. You need to account for the $90 that was spent. After thinking about it, you come up with the following list:
Say you go to the mall with $100, and you come back with only $10. You need to account for the $90 that was spent. After thinking about it, you come up with the following list:
Gas ($15); Sandwich, fries, and a drink ($8); Lost ($5); New clothes ($62)
So you spent $62 on something useful - the clothes - but you spent additional money for other things that were necessary for your trip to the mall.
Self Check
Instructions: Identify the useful energy output(s) and undesirable energy output(s) in the energy conversion devices below. Enter your answers in the fields provided, and click the "Check" button to check your work.
Self Check:
Energy Conversion Devices
For each of the following examples, determine the types of useful energy and undesired energy for the given energy converter.
Example 1: Lawnmower with a chemical energy input. (Hint: How do you know when your neighbor is mowing the lawn?)
Example 2: Car with a chemical energy input. (Hint: Think about mufflers, tires, and generator.)
Example 3: Television with an electrical energy input. (Hint: Have you ever felt the back of your TV after it has been on for a few hours?)
Example 4: Desktop computer with an electrical energy input. (Hint: What’s in your tower and why?)
Answers:
Example 1: The useful energy for a lawnmower is mechanical, while the undesired energy is thermal (heat) and radiation (noise).
Example 2: The useful energy for a car is mechanical, while the undesired energy is thermal or heat (tail pipe).
Example 3: The useful energy for a TV is radiation (light and sound) and the undesirable energy is heat (from circuits).
Example 4: The useful energy for a computer is radiation (light and sound) and the undesirable energy is heat (circuits – electrons moving through system) and mechanical (fan for cooling).
Efficiency of Energy Conversion Devices
Efficiency is the useful output of energy. To calculate efficiency, the following formula can be used:
Example 1
An electric motor consumes 100 watts (a joule per second (J/s)) of power to obtain 90 watts of mechanical power. Determine its efficiency.
Solution:
Input to the electric motor is in the form of electrical energy, and the output is mechanical energy.
Using the efficiency equation:
Or efficiency is 90%.
Caution!
This is a simple example because both variables are measured in Watts. If the two variables were measured differently, you would need to convert them to equivalent forms before performing the calculation.
Practice Problem
Use the following link to generate a random practice problem similar to the Practice 1 example.
The previous example about an electrical motor is very simple because both mechanical and electrical power is given in Watts. Units of both the input and the output have to match; if they do not, you must convert them to similar units.
Example 2
The United States' power plants consumed 39.5 quadrillion Btus of energy and produced 3.675 trillion kWh of electricity. What is the average efficiency of the power plants in the U.S.?
Solution:
Total Energy input = 39.5 x 10^15 Btus and the Useful energy output is 3.675 x 10^12 kWh. Recall that both units have to be the same. So we need to convert kWh into Btus. Given that 1 kWh = 3412 Btus:
Step 1
Therefore:
Step 2
Use the formula for efficiency.
Practice 2
The United States' power plants consumed 39.5 quadrillion Btus of energy and produced 3.675 trillion kWh of electricity. What is the average efficiency of the power plants in the U.S.?
Practice Problem
Use the following link to generate a random practice problem similar to the Practice 2 example.
Energy Efficiencies
Energy efficiencies are not 100%, and sometimes they are pretty low. The table below shows typical efficiencies of some of the devices that are used in day to day life:
| Device | Efficiency |
|---|---|
| Electric Motor | 90 % |
| Home Gas Furnace | 95 % |
| Home Oil Furnace | 80 % |
| Home Coal Stove | 75 % |
| Steam Boiler in a Power Plant | 90 % |
| Overall Power Plant | 36 % |
| Automobile Engine | 25 % |
| Electric Bulb: Incandescent | less than 10 % |
| Electric Bulb: Fluorescent | 60 % |
| Electric Buld: LED | 90 % |
From our discussion on national and global energy usage patterns in Lesson 2, we have seen that:
- about 40% of the US energy is used in power generation;
- about 27% of the US energy is used for transportation.
Yet the energy efficiency of a power plant is about 35%, and the efficiency of automobiles is about 25%. Thus, over 62% of the total primary energy in the U.S. is used in relatively inefficient conversion processes.
Why are power plant and automobile design engineers allowing this? Can they do better?
There are some natural limitations when converting energy from heat to work.
Measuring Thermal Energy
Thermal energy is energy associated with random motion of molecules. It is indicated by temperature, which is the measure of the relative warmth or coolness of an object.
A temperature scale is determined by choosing two reference temperatures and dividing the temperature difference between these two points into a certain number of degrees.
The two reference temperatures used for most common scales are the melting point of ice and the boiling point of water.
- On the Celsius temperature scale, or centigrade scale, the melting point is taken as 0°C and the boiling point as 100°C, with the difference between them being equal to 100 degrees.
- On the Fahrenheit temperature scale, the melting point is taken as 32°F and the boiling point as 212°F, with the difference between them being equal to 180 degrees.
It is important to realize, however, that the temperature of a substance is not a measure of its heat content, but rather, the average kinetic energy of its molecules resulting from their motions.
Try This!
Below is a 6-ounce cup with hot water and a 12-ounce cup with hot water at the same temperature.
- Do they have the same heat content?
- Do they have the same amount of energy?
Instructions: Click the play button to obtain a magnified view of what is happening. Draw your conclusions, enter your answer in the text field provided, and then click the link below the video to check your answer. (Note: The animation has no audio.)
Try This: Measuring Thermal Energy
A six ounce cup and a twelve ounce cup are both filled with 85 degree water.
Conclusion: They do NOT have the same heat content. Since water in the two cups is at the same temperature, the average kinetic energy of the molecules in the cups is the same; however, the 12 ounce cup has twice as many molecules when compared with the 6 ounce cup and thus has the greater total motion or heat energy.
Kelvin Scale
When water molecules freeze at 0°C, the molecules still have some energy compared to ice at -50°C. In both cases, the molecules are not moving, so there is no heat energy.
So what is the temperature at which all the molecules have absolutely zero energy? A temperature scale can be defined theoretically, for which zero degree corresponds to zero average kinetic energy. Such a point is called absolute zero, and such a scale is known as an absolute temperature scale. At absolute zero, the molecules do not have any energy.
The Kelvin temperature scale is an absolute scale having degrees the same size as those of the Celsius temperature scale. Therefore, all the temperature measurements related to energy measurements must be made on Kelvin scale.
You can convert a temperature in Celsius (c) to Kelvin (k) with this formula:
You can also change a temperature in Kelvin to Celsius:
To make calculations for this class easier, you may round off and use just 273 in your conversions.
Try This!
Instructions: Click the "Play" button below and notice what happens to the ice cube. Answer the questions that follow based on your observations. (Note: The animation has no audio.)
Heat Engines
Energy conversions occurring in an automobile are illustrated below:
Any device that converts thermal energy into mechanical energy—such as an automobile or a power plant—is called a heat engine. In these devices, high temperature heat (thermal energy) produced by burning a fuel is partly converted to mechanical energy to do work and the rest is rejected into the atmosphere, typically as a low temperature exhaust.
The Carnot Efficiency
A general expression for the efficiency of a heat engine can be written as:
We know that all the energy that is put into the engine has to come out either as work or waste heat. So work is equal to Heat at High temperature minus Heat rejected at Low temperature. Therefore, this expression becomes:
Where, QHot = Heat input at high temperature and QCold= Heat rejected at low temperature. The symbol (Greek letter eta) is often used for efficiency this expression can be rewritten as:
The above equation is multiplied by 100 to express the efficiency as percent.
French Engineer Sadi Carnot showed that the ratio of QHighT to QLowT must be the same as the ratio of temperatures of high temperature heat and the rejected low temperature heat. So this equation, also called Carnot Efficiency, can be simplified as:
Note: Unlike the earlier equations, the positions of Tcold and Thot are reversed.
The Carnot Efficiency is the theoretical maximum efficiency one can get when the heat engine is operating between two temperatures:
- The temperature at which the high temperature reservoir operates ( THot ).
- The temperature at which the low temperature reservoir operates ( TCold ).
In the case of an automobile, the two temperatures are:
- The temperature of the combustion gases inside the engine ( THot ).
- The temperature at which the gases are exhausted from the engine ( TCold ).

It's like taxes. The more money you earn (heat), the more money is taxed (cold), leaving you with less money to take home (efficiency). However, if you could earn more money (heat) and find a way to have less taxes taken out (better engine material), you would have more money to take home (efficiency).
Below is a table showing two temperature scales. The scale labeled "HOT," shows the range of temperatures for the combustion of gases in a car engine. The scale labeled "COLD," shows the range of temperatures at which gases are exhausted from the car engine.

Instructions: Look carefully at the efficiency numbers in the body of the table. How do the Hot and Cold temperatures' effect on the efficiency.
| Hot 500°C |
Hot 600°C |
Hot 700°C |
Hot 800°C |
Hot 900°C |
Hot 1,000°C |
Hot 1,500°C |
Hot 2,000°C |
|
|---|---|---|---|---|---|---|---|---|
| Cold 150°C |
45 | 52 | 57 | 61 | 64 | 67 | 76 | 81 |
| Cold 125°C |
49 | 54 | 59 | 63 | 66 | 69 | 78 | 82 |
| Cold 100°C |
52 | 57 | 62 | 65 | 68 | 71 | 79 | 84 |
| Cold 75°C |
55 | 60 | 64 | 68 | 70 | 73 | 80 | 85 |
| Cold 50°C |
58 | 63 | 67 | 70 | 72 | 75 | 82 | 86 |
| Cold 25°C |
61 | 66 | 69 | 72 | 75 | 77 | 83 | 87 |
Answer the following questions based on the information in the Car Engine Efficiency table above.
Example
For a coal-fired utility boiler, the temperature of high pressure steam (Thot)would be about 540°C and Tcold, the cooling tower water temperature, would be about 20°C. Calculate the Carnot efficiency of the power plant:
Solution:
Carnot efficiency depends on high temperature and low temperatures between which the heat engine operates. We are given both temperatures. However, the temperatures need to be converted to Kelvin:
Practice
For a coal fired utility boiler, the temperature of high pressure steam would be about 540 degrees C and Tcold, the cooling tower water temperature, would be about 20 degrees C. Calculate the Carnot efficiency of the power plant.
Step 1
Convert the high and low temperatures from Celsius to Kelvin:
Step 2
Determine the efficiency using the Carnot efficiency formula:
From the Carnot Efficiency formula, it can be inferred that a maximum of 64% of the fuel energy can go to generation. To make the Carnot efficiency as high as possible, either Thot should be increased or Tcold (temperature of heat rejection) should be decreased.
Practice Problem
Use the following link to generate a random practice problem.
Entropy and Quality of Energy
Let’s look at an example of how temperature differences are used to generate power. Power plants convert chemical energy into electrical power. Here is a video overviewing the operation of a geothermal energy system, a classic thermal power generation plant.
You may have relaxed in a natural hot springs pool.
Or seen the old faithful geyser blasting hot water into the air in yellowstone national park. But have you ever thought of where all that heat comes from?
Well, it comes from deep beneath the surface of the earth -- and it's called geothermal energy...
And we can use it to generate clean renewable electricity. Ok, here's how geothermal works.
Heat from the earth's crust warms water that has seeped into underground reservoirs. When water becomes hot enough it can break through the earth's surface as steam or hot water. This usually happens where the earth's crust or 'plates' meet and shift.
In the past, taking advantage of geothermal energy was limited to areas where hot water flowed near the surface. But, as geothermal technologies advance, we can leverage even more of these natural renewable energy sources. Engineers have developed a few different ways to produce power from geothermal wells drilled into the ground.
Have a look at this. It's a dry steam geothermal power plant and it's the most common type of geothermal technology used today... Underground steam flows directly to a turbine to drive a generator that produces electricity. Pretty straightforward.
Another geothermal technology is called a flash steam power plant. A pump pushes hot fluid into a tank at the surface, where it cools. As it cools the fluid quickly turns into vapor-- or "flash" vaporizes. The vapor then drives a turbine -- and powers a generator.
A binary cycle plant works differently.
It uses two types of fluid. Hot fluid from underground heats a second fluid, called a heat transfer fluid, in a giant heat exchanger. The second fluid has a much lower boiling point than the first fluid and so it 'flashes' into vapor at a lower temperature. When the second fluid flashes... It spins a turbine that drives a generator.
The environmental benefits of this clean, round-the-clock renewable energy source are substantial: low emissions, small physical footprint, and minimal environmental impact. The few byproducts that can come up are often re-injected underground.
Geothermal energy can also help recycle wastewater. In california, wastewater from the city of santa rosa is injected into the ground to generate more geothermal energy.
Some plants do produce solid waste, but that solid waste may contain minerals that we can remove and sell... Which lowers the cost of this energy source.
The u.s. geological survey estimates that untapped geothermal resources in the united states, if developed, could supply the equivalent of 10% of today's energy needs. In fact, electricity generated by geothermal energy already provides about 60% of the power along the northern california coast...
From the golden gate bridge to the oregon state line.
Geothermal energy... ...helping to push america toward energy independence, and a clean, renewable way to meet our growing energy demands...
Below are two temperature scales. The scale labeled "HOT," shows the range of temperatures for the combustion of gases in a power plant. The scale, "COLD," shows the range of temperatures at which gases are exhausted from the power plant.

Instructions: Look carefully at the efficiency numbers in the body of the table. How do the Hot and Cold temperatures' effect on the efficiency.
| Hot 350°C |
Hot 400°C |
Hot 500°C |
Hot 600°C |
Hot 700°C |
Hot 800°C |
Hot 900°C |
Hot 1,000°C |
|
|---|---|---|---|---|---|---|---|---|
| Cold 300°C |
8 | 15 | 26 | 34 | 41 | 47 | 51 | 55 |
| Cold 250°C |
16 | 22 | 32 | 40 | 46 | 51 | 55 | 59 |
| Cold 200°C |
24 | 30 | 39 | 46 | 51 | 56 | 60 | 63 |
| Cold 150°C |
32 | 37 | 45 | 52 | 57 | 61 | 64 | 67 |
| Cold 100°C |
40 | 45 | 52 | 57 | 62 | 65 | 68 | 71 |
Answer the following questions based on the information in the Power Plant Efficiency table above.
Overall Efficiency
Calculating Overall Efficiency
Using the energy efficiency concept, we can calculate the component and overall efficiency:
Here the electrical energy is given in Wh and Chemical Energy in Btus. So Wh can be converted to Btus knowing that there are 3.412 Wh in a Btu.
This overall efficiency can also be expressed in steps as follows:
Applying this method to the above power plant example:
It can be seen that the overall efficiency of a system is equal to the product of efficiencies of the individual subsystems or processes. What is the implication of this?
Steps of Overall Efficiency
We have been looking at the efficiencies of an automobile or a power plant individually. But when the entire chain of energy transformations is considered—from the moment the coal is brought out to the surface to the moment the electricity turns into its final form—true overall efficiency of the energy utilization will be revealed. The final form at home could be light from a bulb or sound from a stereo. The series of steps as shown in the figure below are: 1) Production of coal (Mining), 2) Transportation to power plant, 3) Electricity generation, 4) Transmission of electricity, and 5) Conversion of electricity into light (Use).
We know what the cumulative efficiency is actually. We looked at that in the previous diagram. We talked about it. On this diagram, we are looking at cumulative or overall efficiency for a qualified power plant. Basically, in the ground, we have coal, right? We have to bring the coal out to the surface. That step is called mining. Obviously, we need to spend some energy to bring the coal from the ground to the surface. So mining by itself has some kind of, let's say, about 95% efficient. Or that step is 95% efficient. In other words, if we have 100 units in the ground, and by the time this energy comes out to the surface, we will be left with only 95 units. Because five units, we will be spending in operating the equipment and in bringing the coal from the ground to the surface. Now, this 95 obviously is not readily available, because we need to take these 95 BTUs to a power plant. So the trucks have to use some energy to take these 95 BTUs all the way up to the power plant. So when it reaches the power plant, these 95 units may turn out to be 90 units. In other words, we will be spending about five units in the transportation. So once we put in 90 units into a power plant, which is roughly about by now 33% efficient or 35% efficient, what this tells us is when we put in 90 units into the power plant, the output from the power plant in the form of electricity is only 30 units. So now, at this point, we have only 30 units left over when we started our business with 100 units. Now, these 30 units are transported through these high voltage lines to a user. By the time you get to the user, we're talking about a unit or two lost. All right? And now, when we have about, let's say for simplicity purposes we will still have about 29 units or 30 units. And as you all know, the efficiency of a light bulb is notoriously low. It's about 5% efficient. Which means that out of these 29 units or 30 units that we will get into the home, only 5% of that, or 1.5 units are converted, really, to the light. So we started off with 100, and we ended up with 1.5 units of light. So that means the overall efficiency is 1.5 divided by 100. Both are BTUs here. So the overall efficiency is only 1.5%. That is pathetically low. Which means to use 1.5 units of light, we are taking from Mother Earth 100 units. And along the way, we are dumping about 98.5 units of energy during various steps of conversion processes, and we're using 1.5 BTUs and 1.5%. That is the message.
Efficiency of a Light Bulb
If the efficiency of each step is known, we can calculate the overall efficiency of production of light from coal in the ground. The table below illustrates the calculation of overall efficiency of a light bulb.
| Step | Step Efficiency | Cumulative Efficiency or Overall Efficiency |
|---|---|---|
| Extraction of Coal | 96% | 96% |
| Transportation | 98% | 94% = (0.96 x 0.98) * 100 |
| Electricity Generation | 35% | 33% = (0.94 x 0.35) * 100 |
| Transmission of Electricity | 95% | 31% = (0.33 x 0.95) * 100 |
| Lighting: Incandescent Bulb |
5% | 1.6 % = (0.31 x 0.05) * 100 |
| Lighting: Fluorescent Bulb |
60% | 18 % = (0.31 x 0.60) * 100 |
Efficiency of an Automobile
A similar analysis on automobile efficiency is shown in the Figure below.
The table below shows that only about 10% of the energy in the crude oil in the ground is in fact turned into mechanical energy moving people.
| Step | Step Efficiency | Cumulative Efficiency or Overall Efficiency |
|---|---|---|
| Extraction of Crude | 96% | 96% |
| Refining | 87% | 84% |
| Transportation | 97% | 81% |
| Engine | 25% | 20% |
| Transmission | 50% | 10% |
Review and Extra Resources
Review
Please watch the 5:30 Lesson 3 Review below:
Hello everyone.
This is our review session for uh chapter three in this course where we're learning about energy efficiency and energy uh conversion processes.
So the real key of this chapter here is this one equation. And this is the one equation that i will not provide in the exams or in the sheets because i want you to memorize it because it is at the heart of this whole of this whole uh course. So useful energy out over total energy in is our energy efficiency.
Now remember the total energy in will always equal the total energy out, but many of it will be in forms that are not useful. So what that means is essentially we consume some primary energy source and then a fraction of that total energy we're putting in goes to something that we want it to. okay and it's always going to be less.
So whether we are moving the wheels on our car, we're trying to heat something up so that we can cook it the total energy in and that usefUl portion we're using to do some work it will be less.
Okay and so in each case we want to identify because there will be many energy outputs right and that's the first law of thermodynamics that the total energy in must always equal the total energy out. You cannot create or destroy energy and that's what's really encompassed in this first bullet here. All the energy we put in does not come out in the desired form. No such thing. An important thing to note when using this equation is that the energy should have the same units.
So you can't put joules in here and then say quads or btus or calories in here because the unit conversions will give you strain strange values for this efficiency. So it's really important when you use this to make sure that the units in both the top and the bottom match.
Another important note is that temperature of a substance is not a measure of its heat content. That's that's actually something that's energy related but instead it's the average kinetic energy of the molecules in their motion. Okay, one of the classic examples that was used long long ago to try to identify this equation and to describe it and what started the industrial revolution is the heat engine which is essentially a device that converts thermal energy into mechanical energy. So this comes up in our everyday lives well it used to come up more but as batteries and electrical energy conversion processes are coming in maybe it'll come up a bit less as we turn off of fossil fuels but still there are a lot of classic examples we can see.
Cars for example we take a gasoline which is chemical energy we then burn it to create thermal energy and then that thermal energy ends up spinning uh the wheels on our car or the blades on our lawn mowers. Right and it also works in a larger scale for coal and natural gas power plants when they're moving a turbine.
So these heat engines are described by the carnot efficiency which is a classic equation to tell us the limit of the absolute most energy we can get out of the thermal energy source. Right um to use this equation properly all the temperatures must be in kelvin. This is really important when you're ever dividing temperatures kelvin should always be used. And then you can see some clear relations with carnot efficiency that lets you know how a heat engine can be more or less efficient. A lot of that has to do with the temperature temperatures that are you're using right.
And so many of these principles are the key uh the key building blocks behind the workings of a power plant and as we had mentioned before right this thermal energy to mechanical energy isn't really one step but in fact in a total power plant there are many steps. Right so we have to go often from chemical energy to thermal energy to mechanical energy and then from mechanical energy we go to electrical energy and that's what we use to power our laptops and keep the lights on. And the way that we account for that is we essentially use our energy efficiency equation and we do that for each of those steps and then we multiply the efficiency of those steps together and that tells us our overall efficiency of the process. Right so what is the efficiency of going from chemical to thermo again it will never be a hundred percent so some will be we lost. And then from that useful thermo how much is going into mechanical and etc etc.
Okay so please take some time to review each of these key key points in this lecture and good luck on the quiz.
Okay.
Review Sheet Lesson 3 – Energy Efficiency
- Energy Conversion
- All the energy that we put in may not come out in the desired form
- Efficiency = Useful Energy Output / Total Energy Input
- Both energies must in the same units
- The temperature of a substance is not a measure of its heat content, but rather, the average kinetic energy of its molecules resulting from their motions
- Heat Engine
- Device that converts Thermal energy into Mechanical energy
- Carnot Efficiency
- All temperatures must be in Kelvin
- As Tlow decreases, efficiency increases. As Tlow increases, efficiency decreases
- As Thot decreases, efficiency decreases. As Thot increases, efficiency increases
- Workings of a Power plant
- Overall Efficiency = product of step efficiencies
Test Yourself
The questions below are your chance to test and practice your understanding of the content covered in this lesson. In other words, you should be able to answer the following questions if you know the material that was just covered! If you have problems with any of the items, feel free to post your question on the unit message board so your classmates, and/or your instructor, can help you out!
- A heat engine has Carnot efficiency of 30%. Useful output from the engine is 1000J. How much heat is wasted?
- How can we improve the Carnot efficiency of a heat engine by changing the hot and cold reservoir temperatures?
- Most of the energy conversion devices that we use in our day-to-day life can be classified as Heat Engines. Give two examples.
Extra Resources
For more information on topics discussed in Lesson 3, see these selected References:
- Hinrichs, R. A., “Energy,” Saunders College Publishers, Philadelphia, PA, 1992.
- Aubrecht, G. L., “Energy,” Prentice Hall, Inc., Englewood Cliffs, NJ, 1995.
- Fay, J.A. and Golomb, D. S., “Energy and the Environment,” Oxford University Press, New York, NY, 2002.
- Christensen, J. W., “Global Science: Energy Resources Environment."
Lesson 3 Deliverables
Deliverable 1
You must complete a short quiz that covers the reading material in lesson 3. The Lesson 3 Quiz, can be found in the Lesson 3: Energy Efficiency module in Canvas. Please refer to the Calendar in Canvas for specific time frames and due dates.







 >
>