Lesson 1: Introduction to Petroleum Refining and Crude Oil Composition
Lesson 1 Overview
Lesson 1 Overview
Video: FSC 432 Lesson 1 (2:04)
Hello. In this lesson, we will go over the market drivers for Petroleum Refining Industry, an overview of refinery processes and refinery products, and the chemical constitution of crude oil. Obviously, the principle driver for Petroleum Refining Industry is economics that is now very closely tied to environmental regulations as well. So we need to look into the supply and demand picture, whether it is for the crude oil or the refined products such as fuels, gasoline, diesel, jet fuel, et cetera, and the environmental regulations on the compositions of these fuels, as well as on the operation of the refinery, and the flexibility of the refinery to respond to the demand shifts or supply shifts on either side of this equation.
And we go over finally the chemical constitution of this very complex feedstock, crude oil, and how we can in essence better understand how different each crude oil is, and what our refiners can do to adjust the processes to accommodate these differences in the crude oil circle. A recent development in the United States increased production of shale gas, which also produces some liquid byproducts that are entering the refinery as alternative feedstock. We need to really understand how these feeds could be refined to give us a conventional set of products that we produce in the refinery. So I look forward to seeing you in these lessons and go through the activities.
Overview
Petroleum provides the largest fraction of primary energy supply in the U.S. and in the world [Figure 1.1,eia1]. Resource consumption patterns shown in Figure 1.1 reflect major epochs in human history, such as The Industrial Revolution, ushering in the rapid increase in coal consumption. Petroleum trace, for example, marks the mass production of automobiles with the introduction of Model T by Ford, world wars, supply crises of 1973 and 1979 and the economic recession in 2008. Transportation of people and goods in many parts of the world depends almost completely on petroleum fuels, such as gasoline, jet fuel, diesel fuel, and marine fuel. Apart from the fuels, materials that are necessary for operating the combustion engines of cars, trucks, planes, and trains also come from petroleum. These materials include lubricating oils (motor oils), greases, tires on the wheels of the vehicles, and asphalt to pave the roads for smooth rides in transportation vehicles. All petroleum fuels and many materials are produced by the processing of crude oil in petroleum refineries. Petroleum refineries also supply feedstock to the petrochemicals and chemical industry for producing all consumer goods from rubber and plastics (polymers) to cosmetics and medicine. Only ten percent of petroleum consumption, the portion that is not used for transportation or other energy outlets, is sufficient to manufacture all the materials used in human economy, with the exception of those derived from wood or minerals.

Figure 1.1 is showing the history of energy consumption of the U.S. and the source of the energy. For nearly the first 100 years, the U.S. used only wood as our energy source until about the mid 1800’s. Afterward they started using more of what we think of as our standard energy materials such as coal and petroleum. The 20th century is when sources we now consider alternative started to appear such as hydraulic, nuclear, solar and natural gas.
The petroleum industry consists of two separate operations: Upstream and Downstream Operations. Upstream operations involve exploration of new oil reserves, development of oil fields, constructing the well-head and crude oil production facilities. Downstream operations cover processing of crude oil in petroleum refineries to produce liquid and gaseous fuels and materials for the market. This course addresses petroleum refining to review how a variety of physical processes and chemical reactions in separate refinery units are integrated to process compliant fuels and materials.
Learning Outcomes
By the end of this lesson, you will be able to:
- recognize the significance of petroleum fuels in the U.S. energy supply;
- express the overall objectives of petroleum refining;
- identify the economic and environmental drivers of petroleum refining;
- describe the overall approach to petroleum refining and categorize refinery processes and products;
- portray chemical constitution of petroleum.
What is due for Lesson 1?
This lesson will take us one week to complete. Please refer to the Course Syllabus for specific time frames and due dates. Specific directions for the assignments below can be found on the Assignment page within this lesson.
| Readings | J. H. Gary, G. E. Handwerk, Mark J. Kaiser, Chapter 1, pp. 1-12; Chapter 3, pp. 62-65 |
|---|---|
| Assignments | For your information, review the most recent supply of petroleum fuels from the data given at U.S. Energy Information Administration [3] (eia.gov) and research how petroleum refining addresses the environmental concerns from the combustion of petroleum fuels in internal combustion engines. |
Questions?
If you have any questions, please post them to our Help Discussion (not email), located in Canvas. I will check that discussion forum daily to respond. While you are there, feel free to post your own responses if you, too, are able to help out a classmate.
Market Drivers for the Refining Industry
Market Drivers for the Refining Industry
Markets and demand for refinery products depend on the dynamics of a global economy. It is generally agreed that oil and gas will continue to be the primary energy resource in the U.S. and world economies for decades to come. Because of the projected increase in the production of oil in tight formations, the United States is expected to become an exporter of petroleum products and crude oil after decades of being an importer (Figure 1.2, EIA Annual 2013, eia.gov). Petroleum fuels will continue to dominate the transportation sector, but the following trends should be noted:
- increasing fuel economy of vehicles (offset by increasing number of vehicles and miles driven);
- more strict environmental regulations with demand for cleaner fuels;
- biofuels as additives (e.g., ethanol, biodiesel) or alternative fuels in niche markets (jet fuel from algae);
- demand for high-quality, high-performance fuels

Figure 1.2 is showing both the history of U.S. crude oil production since 1990 as well as the prediction of U.S. crude oil production until the year 2040. Since 1990, we have been seeing a decline in oil production from Alaska, offshore drilling, and lower 48 offshore production until 2010 and then those sources are predicted to level off. After 2010 it is predicted that oil production from tight oil will continue to increase.
Competitive forces in the global economy lead to joint ventures and mergers and shutting down of inefficient refineries, or shutting down of processing units with low efficiency within refineries. Figure 1.3 shows the changes in the refinery capacity and number of refineries in the U.S. since 2000. The increasing refining capacity, with the decreasing number of refineries, results in the closing down of small inefficient refineries while expanding the large refineries.

Regarding the global competition, the technological advancement addresses the degrading quality of crude oils to produce cleaner and higher quality petroleum fuels. On the supply side, there is the increasing abundance of natural gas liquids (ethane, propane, n-butane, and isobutane) due to increased shale gas production in the U.S. and elsewhere. These liquids enter refineries as new feedstock in addition to crude oil supply.
Refineries need process improvements to advance their capabilities to deal with the changing crude oil base and changing environmental regulations. These improvements in refinery processes would need to create and use, for example:
- new catalysts and new chemistry;
- more sophisticated process modeling and computational methods;
- more effective use of computers in refinery management;
- online monitoring and property measurements;
- new materials to reduce maintenance and extend the useful life of the equipment.
Concerns for efficiency include running a refinery efficiently and producing fuels that will burn efficiently in the combustion engines, as follows:
- Efficiency of refinery processes
- Minimize waste and optimize the yield and properties of the refinery products to obtain maximum value from the crude oil.
- Increase the energy efficiency of each unit in the refinery.
- Fuel economy in internal combustion engines
- Produce high-performance fuels for efficient operation of combustion engines.
An Overview of Refinery Products and Processes
An Overview of Refinery Products and Processes
Considering the market drivers just reviewed along the small profit margins that are often usually associated with petroleum refinery products, refineries should carefully select the crude oil feedstock and configure the refinery processes such that they produce the desirable petroleum products at the lowest cost.
In the U.S. refineries, a principal focus is on the production of gasoline because of high demand. Diesel fuel is the principal refinery product in most other parts of the world. Figure 1.4 shows a typical distribution of products from a barrel of crude oil in a U.S. refinery. Distillation process separates the crude oil into boiling point fractions. The liquefied petroleum gas (LPG) constitutes the lowest boiling point (most volatile) product from a refinery and higher boiling fractions lead to most desirable distillate liquids, such as gasoline, jet fuel, diesel fuel, and fuel oil in the increasing order of boiling points, while asphalt is made from the residual fraction remaining after distillation.
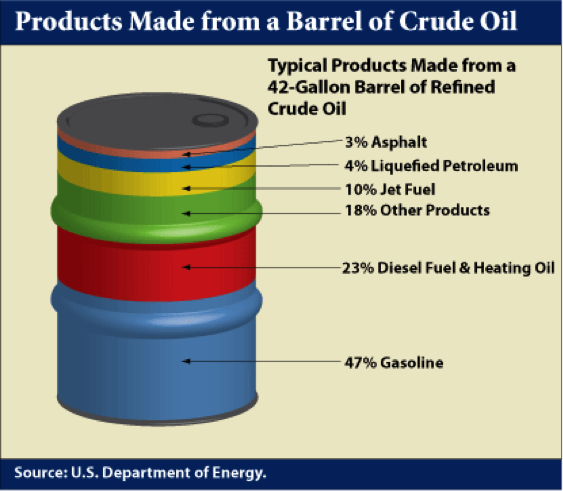
Products Made from a Barrel of Crude Oil
-
47% Gasoline
-
23% Diesel Fuel & Heating Oil
-
18% Other Products
-
10% Jet Fuel
-
4% Liquefied Petroleum
-
3% Asphalt
The following animation shows a refinery flow chart indicating some of the major refinery processes and refinery products. Note that the distillation process (Fractionation Tower) separates crude oil into a number of distillate fractions that are sent as feedstocks to different processes, some of which are interconnected. It is also important to recognize that petroleum refining not only produces transportation fuels and fuels for space heating or industrial furnaces, but also produces materials needed for the operation of the combustion engines and paving the roads for vehicles to travel on.
Video: FSC 432 Refinery Flow Chart (4:12)
Here, we will build a simple refinery flow chart. On the left, you see the crude oil feed to the refinery. On the right hand side, the major refinery products, going from the lightest to the heaviest. Starting with gasoline, jet fuel and kerosene, heating and diesel fuels, industrial fuel oil, waxes, lubricating oils, greases, asphalt, and petroleum coke would be the heaviest product, which will be a solid obtained from a refinery.
The crude oil is fed to the fractionating tower that we call the distillation calm. We separate the crude oil into various boiling fractions. And these fractions are fed to the processes, the downstream, which are vapor recovery unit, also forming alkylation, catalytic cracking, extraction, coking, dewaxing, grease manufacturing, treating and blending, among others and there's additional processing there as well. So, we essentially will connect to crude oil through these processes to the final product.
Now, please note that some of the refinery units are connected. If you look at vapor recovery unit connected to catalytic cracking, that is also connected to coking. And at the top is ultraforming. Now, these processes all produce gasoline and light to hydrocarbons, like LPG, from different bonding fractions of crude oil coming from the distillation columns. That's why they're linked on this diagram.
Let's follow what happens to different distillation fractions coming from the distillation column. First, the vapor product from the top is sent to the vapor recovery unit, and separates into a gasoline and LPG-- that's liquified petroleum gas. You can see the ultraforming we call now, the process of Catalytic Reforming is involved to make a high-octane gasoline.
You can see that additional processing is also needed to remove sulfur out from these products. LPG as well as gasoline. We should note that catalytic cracking can also produce jet fuel. As you can see the arrow from cat cracking touching the jet fuel point. And catalytic cracking also produces feed stocks for the alkylation unit to produce additional high octane gasoline.
As we go down to the distillation column, we are now into the vacuum distillation territory, and the product from vacuum distillation would go through extraction, dewaxing, and various treating and blending to produce lubricating oils as well as waxes and greases.
We are now at the bottom of the vacuum distillation column, the vacuum distillation residue can do various things with this fraction versus coking. It's a very severe thermal cracking process, which leads to petroleum coke as a byproduct. Refineries use coking to produce more jet fuel gasoline and then LPG. Petroleum coke is just a byproduct.
The vacuum distillation residue could be treated in a deasphalting process to produce asphalt. So, again, as a byproduct, the principal product from the deasphalting called the deasphalted oil could be used to making a lighter, hydrocarbons, fuels, and chemicals from this fraction.
And this pretty much completes building off a very simple refinery flow chart.
Figure 1.5 indicates that chemical constitution and physical properties of crude oils are important parameters that guide the refinery configurations. The refining processes can be divided into four groups, as indicated. While the separation processes involve just physical phenomena, the conversion, finishing, and support processes require chemical changes, i.e., breaking chemical bonds to modify the molecular structure of the feedstocks. These changes are necessary to produce the fuels and materials in accordance with industrial/commercial specifications.

This is a classification of refining processes and the types of refinery products, shown by a flow chart. The flow chart starts with crude oil. Above crude oil chemical constitution is written and below physical properties are written. Crude oil leads to the refining process including separation, conversion, finishing and support. From there it goes to products including fuels, petrochemicals and materials. Underneath this is written specifications.
Figure 1.6 (progressive image, 25 seconds) shows a more detailed refinery block diagram to show how different processes are integrated for producing the desired fuels and materials.

Separation processes, such as distillation, dewaxing, and deasphalting make use of the differences in the physical properties of crude oil components to separate groups of hydrocarbon compounds or inorganic impurities, whereas conversion processes cause chemical changes in the hydrocarbon composition of crude oils. For example, Fluid Catalytic Cracking process breaks chemical bonds in long-chain alkanes to produce shorter chain alkanes to produce gasoline from higher boiling gas oil fractions. Finishing processes involve hydrotreating to remove heteroatoms (S, N, and metals) and product blending to produce fuels and materials with desired specifications and in compliance with environmental and government regulations. Finally, supporting processes provide the recovery of the removed heteroatoms or heteroatom compounds, production of the hydrogen necessary for conversion and hydrotreating processes, and effluent water treatment systems.
Knowledge Check
Why is diesel fuel preferred over gasoline in many countries in the world?
ANSWER: Diesel fuel powers the engines in buses, locomotives, and ships used for public transport. Passenger vehicles fueled by gasoline are the most widely used means of transportation in the U.S.
Chemical Constitution of Crude Oil
Chemical Constitution of Crude Oil
Crude oil contains organic compounds, heteroatom compounds (S,N,O), hydrocarbons (C, H), metals and organic (Ni, V, Fe) and inorganic (Na+, Ca++, Cl-) compounds as listed in Figure 1.7. Compounds that contain only elements of carbon and hydrogen are called hydrocarbons and constitute the largest group of organic compounds found in petroleum. There might be as many as several thousand different hydrocarbon compounds in crude oil. Hydrocarbon compounds have a general formula of CxHy, where x and y are integer numbers.

Triangle labeled Crude Oil. On each corner is:
Inorganic Compounds (Na+, Ca2+, Cl-)
Organic Compounds (Ni, V, Fe)
Organic Compounds
On each side they are labeled:
Between organic and inorganic compounds: Heteroatom compounds (S,N,O)
Between organic and Organic (Ni, V, Fe) compounds: Hydrocarbons (C,H)
Between Organic (Ni, V, Fe) and inorganic compounds: Metals
Hydrocarbons are generally divided into four groups: (1) paraffins, (2) olefins, (3) naphthenes, and (4) aromatics (Figure 1.8). Among these groups, paraffins, olefins, and naphthenes are sometimes called aliphatic compounds, as different from aromatic compounds. The lightest hydrocarbon found as a dissolved gas is methane (CH4), the main component of natural gas. Olefins are not usually found in crude oils, but produced in a number of refining processes.

Image reads:
Essential components of petroleum
-CH compounds based on quadrivalency of carbon atoms
-linked by a single bond (alkanes)
-liked by a double bond (alkenes)
-linked by conjugated double bonds in a ring structure (aromatics)
Saturated Aliphatic HC (n-alkanes or n-paraffins)
-straight chains of C atoms each with 2, 3 H atoms (except CH4)
-general formula: CnH2n+2
-CH3-(CH2)n-(CH3): Ex) n-pentane: CH3-CH2-CH2-CH2-CH3
Aromatic Hydrocarbons
Aromatic Hydrocarbons
Aromatic hydrocarbons are an important series of hydrocarbons found in almost every petroleum mixture from any part of the world. Aromatics are cyclic but unsaturated hydrocarbons with alternating double bonds (Figure 1.12). The simplest aromatic hydrocarbon is benzene (C6H6). The name “aromatic” refers to the fact that such hydrocarbons are commonly fragrant compounds. Although benzene has three carbon-carbon double bonds, it has a unique arrangement of electrons with resonance structures of the double bonds (aromaticity) that allow benzene to be relatively stable. However, benzene is known to be a cancer-inducing compound. For this reason, the amount of benzene allowed in petroleum products such as gasoline or fuel oil is limited by government regulations in many countries. Under standard conditions, benzene, toluene, and xylene are in liquid form whereas higher aromatics such as naphthalene occur as solids in isolation, but dissolve to form a liquid solution with simple aromatics.

Image Shows Cyclic and polyunsaturated hydrocarbons with conjugated double bonds. Specifically:
Benzene: six carbon ring with no side chains
Alkylaromatics:
-Toluene: benzene with a methyl group on carbon 1
-Xylene (meta): benzene with a methyl group on carbons 1 & 3
-Ortho: benzene with a methyl group on carbon 1&2
-Para: benzene with a methyl group on carbon 1&4
Knowledge Check
What constitutes the white crystals of moth balls?
ANSWER: Napthalene! Naphthalene is an effective moth killer because it sublimes (forms a vapor from a solid without going through a liquid state) at room temperature.
Polyaromatic and Hydroaromatic Compounds
Polyaromatic and Hydroaromatic Compounds
Some of the common aromatics found in crude oil and petroleum products are benzene derivatives with attached methyl, ethyl, propyl, or higher alkyl groups. This series of aromatics is called alkylbenzenes, and compounds in this homologous group of hydrocarbons have the general formula of CnH2n-6 (where n ≥ 6). Generally, an aromatic series with only one benzene ring is also called mono- aromatics or mononuclear aromatics. However, heavy petroleum fractions and residues contain unsaturated multirings with many benzene and naphthene rings attached to each other. Such aromatics that exist as solids in isolation are also called polyaromatic hydrocarbons (PAHs) or polynuclear aromatics (PNAs) (Figure 1.13). Heavy crude oils usually contain more aromatics than light crudes. It is common to have compounds with naphthenic and aromatic rings side by side (hydroaromatics, or naphthenoaromatics, Figure 1.13) especially in heavy fractions.
Figure 1.13 shows examples of PAHs, such as anthracene, phenathrene, and pyrene. The configuration of rings in PAHs strongly influences the physical and chemical properties of these compounds. For example, three-ring aromatics anthracene and phenanthrene have significantly different properties. In petroleum, PAHs exist mostly as alkyl substituted ring systems such that the substitutent alkyl groups (e.g., methyl, ethyl) replace (substitute for) the hydrogen atoms on the rings.

Image Reads:
Polyaromatic Hydrocarbons (PAH) or Polynuclear Aromatics (PNA)
Aromatic hydrocarbons containing more than one ring:
-Ex) Naphthalene, Anthracene, Phenanthrene, Pyrene
-Associated with environmental and health problems – toxic compounds
-deactivate catalysts via coking reactions
Hydroaromatics of naphtenoaromatics
-partially saturated PAH; e.g. tetralin (tetrahydronapthalene) – strong H donors
Normally, high-molecular-weight polyaromatics contain several heteroatoms such as sulfur, nitrogen, or oxygen, but these compounds are still called aromatic compounds because their electronic configurations maintain the aromatic character.
Sulfur is the most important heteroatom found in crude oil and refinery products petroleum, and it can be found in cyclic (e.g., thiophenes) and noncyclic compounds such as mercaptans (R-S-H) and sulfides (R-S- R′), where R and R′ are alkyl groups. Sulfur in natural gas is usually found in the form of hydrogen sulfide (H2S). Figure 1.14 shows the types of sulfur compounds in crude oils. The amount of sulfur in a crude oil may vary from 0.05 to 6 % by weight. The presence of sulfur in finished petroleum products is not desirable. For example, the presence of sulfur in gasoline can promote corrosion of engine parts and produce sulfur oxides upon combustion, contributing to air pollution.

Normally, the concentration of the other heteroatom compounds (nitrogen, oxygen, and metals) in crude oils is usually lower than that of the sulfur compounds. Figure 1.15 shows the nitrogen compounds that may be found in crude oils.
Generally, in heavier crude oils the proportions of carbon, sulfur, nitrogen, and oxygen compounds are higher at the expense of hydrogen content. Heavier crude oils also contain organometallic compounds of common nickel and vanadium (Figure 1.16). These compounds are highly corrosive and toxic and should be removed in the refinery. Nickel, vanadium, and copper can also severely affect the activities of catalysts and result in lower quality products. Organometallic compounds tend to concentrate in heavy, or residual fractions of crude oils.


Knowledge Check
What is the principal type of air pollution caused by the emission of sulfur oxides into the atmosphere?
ANSWER: Acid rain, caused by the formation of sulfuric acid through reactions of the sulfur oxides in the atmosphere.
Paraffins
Paraffins
Paraffins are also called alkanes and have the general formula of CnH2n+2, where n is the number of carbon atoms in a given molecule. Paraffins are divided into two groups of normal and isoparaffins. Normal paraffins or normal alkanes are simply written as n-paraffins or n-alkanes, and they are open, straight-chain saturated hydrocarbons. The second group of paraffins is called isoparaffins, which are branched-type hydrocarbons, and they begin with isobutane (also called methylpropane), which has the same closed formula as n-butane (C4H10). Compounds of different structures with the same closed formula are called isomers (Figure 1.9). For example, the open formula for n-butane, n-C4, can be shown as CH3-CH2-CH2-CH3, based on the quadrivalency of the carbon atom, and for simplicity, only the carbon-carbon bonds are drawn and most C-H bonds are omitted, as shown in Figure 1.7 and 1.8 on the previous page. Paraffins are the largest series of hydrocarbons found in petroleum and beginning with the simplest compound, methane.
Under standard conditions of temperature and pressure (STP), the first four members of the alkane series (methane, ethane, propane, and butane) are in gaseous form, and compounds starting from C5H12 (pentane) to n-heptadecane (C17H36) are liquids (constituting large fractions of hydrocarbons found in liquid fuels (e.g., gasoline, jet fuel, and diesel fuel), whereas n-octadecane (C18H38) or heavier compounds exist in isolation as wax-like solids at STP. These heavier paraffins are soluble in lighter paraffins or other hydrocarbons and can be found in diesel fuel and fuel oils. Paraffins from C1 to C40 usually appear in crude oil (heavier alkanes in liquid solution, not as solid particles) and represent up to 20% of crude by volume.
Figure 1.10 shows the statistically possible number of isomers of paraffins that increase exponentially with carbon number, starting with just one isomer for butane, reaching approximately 60,000 for C18 paraffins. Note that the branching in hydrocarbons causes significant changes in physical properties (e.g., boiling point and density, Figure 1.11) and chemical behavior (e.g., octane number, Figure 1.10) of paraffins with the same carbon number. Note in Figure 1.10 that the removal of an H atom from alkanes generates free radicals (reactive species containing unpaired electrons) that are called alkyl species (e.g., methyl formed from methane and ethyl formed from ethane by removing a hydrogen atom) also a radical with an unpaired electron. Also note the nomenclature using alkyl groups to specifically name isoalkanes (e.g., 2,2,4-trimethylpentane to designate a specific iso-octane).

Image Reads:
Boiling point and density increase with increasing # of carbon atoms
Effect is much more pronounced at low carbon #’s
Boiling point of n-alkanes is greater than the boiling point of iso-alkanes with the same # of carbons
-Due to molecular interactions and dispersion forces
Isomers: compounds that have the same chemical formula but different structures and different chemical and physical properties
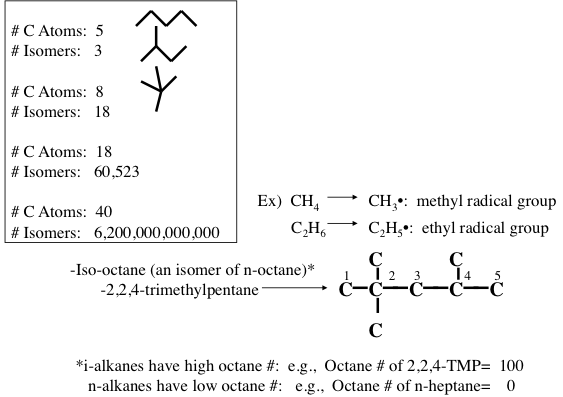
Image Reads
| # of Carbons | # of Isomers |
|---|---|
| 5 | 3 |
| 8 | 18 |
| 18 | 60,523 |
| 40 | 6.2x1012 |
Iso-octane (an isomer of n-octane): 2,2,4-trimethylpentane
-i-alkanes have high octane #’s: e.g. Octane # of 2,2,4-TMP = 100
-n alkanes have low octane #’s: e.g. Octane # of n-heptane = 0
Naphthenes or cycloalkanes are rings or cyclic saturated hydrocarbons with a general formula of CnH2n5H10), cyclohexane (C6H12), and their derivatives such as n-alkylcyclopentanes are normally found in crude oils.

Image Reads:
Cyclic structures (or rings) in all or part of the skeleton.
-e.g cyclohexane
The general formula for single ring compounds: CnH2n
-Boiling points and densities of cycloalkanes are greater than the boiling points on n-alkanes with the same # of carbons
-For example: ethylene (H2C=CH2) and propylene (H2C=CH-CH3); petrochemical feedstocks
Unsaturated Aliphatic HC (alkenes or olefins)
-little or no olefins in crude oils (produced by refinery operations)
A hexagon is a misleading 2-D representation of cyclohexane the actual structure looks more like a boat or a chair
Assignments
Assignment Reminder
Each week, you will be required to do assignments. The assignment for this week is:
For your information (no submission), review the most recent supply of petroleum fuels from the data given at U.S. Energy Information Administration [4] and research how petroleum refining addresses the environmental concerns from the combustion of petroleum fuels in internal combustion engines.
Self-Check Questions
Self-Check Questions
Take a few minutes to answer the five questions below. Use the arrow to go to the next question. When you are ready, click Check to see the solution.
Summary and Final Tasks
Summary
Petroleum, the most important crude oil, consists of a mixture of hydrocarbon compounds including paraffinic, naphthenic, and aromatic hydrocarbons with small amounts of impurities including sulfur, nitrogen, oxygen, and metals. The first process in petroleum refining operations is the separation of crude oil into its major constituents, using distillation to separate the crude oil constituents into common boiling-point fractions. Other separation processes include deasphalting to remove the heaviest fraction of crude oil, asphalt, and dewaxing to remove long-chain n-paraffins called wax.
To meet the demands for high-octane gasoline, jet fuel, and diesel fuel, heavier components of crude oils are converted to gasolines and other distillate fuels. Among the conversion processes are cracking, coking, and visbreaking that are used to break large petroleum molecules into smaller ones. Polymerization and alkylation processes are used to combine molecules smaller than those in gasoline into larger ones to make more gasoline in the refinery. Isomerization and reforming processes are applied to rearrange and reform the structure of hydrocarbons to produce higher-value gasoline components of a similar molecular size.
Finishing processes in a refinery processes stabilize and upgrade petroleum products by hydrogenation and remove undesirable elements, such as sulfur and nitrogen, by hydrotreating processes. Blending of many product streams, to come up with commercial refinery products with the required specifications, also belong to the category of finishing processes.
Lesson Objectives
You should now be able to:
- recognize the significance of petroleum fuels in the U.S. energy supply;
- express the overall objectives of petroleum refining;
- identify the economic and environmental drivers of petroleum refining;
- describe the overall approach to petroleum refining and categorize refinery processes and products;
- portray chemical constitution of petroleum.
Reminder - Complete all of the Lesson 1 tasks!
You have reached the end of Lesson 1! Double-check the to-do list below to make sure you have completed all of the activities listed there before you begin Lesson 2. Please refer to the Course Syllabus for specific time frames and due dates. Specific directions for the assignment below can be found within this lesson.
| Readings | J. H. Gary, G. E. Handwerk, Mark J. Kaiser, Chapter 1, pp. 1-12; Chapter 3, pp. 62-65 |
|---|---|
| Assignments | For your information, review the most recent supply of petroleum fuels from the data given at The U.S. Energy Information Administration website [3] (eia.gov) and research how petroleum refining addresses the environmental concerns from combustion of petroleum fuels in internal combustion engines. |
Questions?
If you have any questions, please post them to our Help Discussion (not email), located in Canvas. I will check that discussion forum daily to respond. While you are there, feel free to post your own responses if you, too, are able to help out a classmate.