Chemical Constitution of Crude Oil
Chemical Constitution of Crude Oil
Crude oil contains organic compounds, heteroatom compounds (S,N,O), hydrocarbons (C, H), metals and organic (Ni, V, Fe) and inorganic (Na+, Ca++, Cl-) compounds as listed in Figure 1.7. Compounds that contain only elements of carbon and hydrogen are called hydrocarbons and constitute the largest group of organic compounds found in petroleum. There might be as many as several thousand different hydrocarbon compounds in crude oil. Hydrocarbon compounds have a general formula of CxHy, where x and y are integer numbers.

Hydrocarbons are generally divided into four groups: (1) paraffins, (2) olefins, (3) naphthenes, and (4) aromatics (Figure 1.8). Among these groups, paraffins, olefins, and naphthenes are sometimes called aliphatic compounds, as different from aromatic compounds. The lightest hydrocarbon found as a dissolved gas is methane (CH4), the main component of natural gas. Olefins are not usually found in crude oils, but produced in a number of refining processes.

Aromatic Hydrocarbons
Aromatic Hydrocarbons
Aromatic hydrocarbons are an important series of hydrocarbons found in almost every petroleum mixture from any part of the world. Aromatics are cyclic but unsaturated hydrocarbons with alternating double bonds (Figure 1.12). The simplest aromatic hydrocarbon is benzene (C6H6). The name “aromatic” refers to the fact that such hydrocarbons are commonly fragrant compounds. Although benzene has three carbon-carbon double bonds, it has a unique arrangement of electrons with resonance structures of the double bonds (aromaticity) that allow benzene to be relatively stable. However, benzene is known to be a cancer-inducing compound. For this reason, the amount of benzene allowed in petroleum products such as gasoline or fuel oil is limited by government regulations in many countries. Under standard conditions, benzene, toluene, and xylene are in liquid form whereas higher aromatics such as naphthalene occur as solids in isolation, but dissolve to form a liquid solution with simple aromatics.

Knowledge Check
What constitutes the white crystals of moth balls?
Polyaromatic and Hydroaromatic Compounds
Polyaromatic and Hydroaromatic Compounds
Some of the common aromatics found in crude oil and petroleum products are benzene derivatives with attached methyl, ethyl, propyl, or higher alkyl groups. This series of aromatics is called alkylbenzenes, and compounds in this homologous group of hydrocarbons have the general formula of CnH2n-6 (where n ≥ 6). Generally, an aromatic series with only one benzene ring is also called mono- aromatics or mononuclear aromatics. However, heavy petroleum fractions and residues contain unsaturated multirings with many benzene and naphthene rings attached to each other. Such aromatics that exist as solids in isolation are also called polyaromatic hydrocarbons (PAHs) or polynuclear aromatics (PNAs) (Figure 1.13). Heavy crude oils usually contain more aromatics than light crudes. It is common to have compounds with naphthenic and aromatic rings side by side (hydroaromatics, or naphthenoaromatics, Figure 1.13) especially in heavy fractions.
Figure 1.13 shows examples of PAHs, such as anthracene, phenathrene, and pyrene. The configuration of rings in PAHs strongly influences the physical and chemical properties of these compounds. For example, three-ring aromatics anthracene and phenanthrene have significantly different properties. In petroleum, PAHs exist mostly as alkyl substituted ring systems such that the substitutent alkyl groups (e.g., methyl, ethyl) replace (substitute for) the hydrogen atoms on the rings.

Normally, high-molecular-weight polyaromatics contain several heteroatoms such as sulfur, nitrogen, or oxygen, but these compounds are still called aromatic compounds because their electronic configurations maintain the aromatic character.
Sulfur is the most important heteroatom found in crude oil and refinery products petroleum, and it can be found in cyclic (e.g., thiophenes) and noncyclic compounds such as mercaptans (R-S-H) and sulfides (R-S- R′), where R and R′ are alkyl groups. Sulfur in natural gas is usually found in the form of hydrogen sulfide (H2S). Figure 1.14 shows the types of sulfur compounds in crude oils. The amount of sulfur in a crude oil may vary from 0.05 to 6 % by weight. The presence of sulfur in finished petroleum products is not desirable. For example, the presence of sulfur in gasoline can promote corrosion of engine parts and produce sulfur oxides upon combustion, contributing to air pollution.

Normally, the concentration of the other heteroatom compounds (nitrogen, oxygen, and metals) in crude oils is usually lower than that of the sulfur compounds. Figure 1.15 shows the nitrogen compounds that may be found in crude oils.
Generally, in heavier crude oils the proportions of carbon, sulfur, nitrogen, and oxygen compounds are higher at the expense of hydrogen content. Heavier crude oils also contain organometallic compounds of common nickel and vanadium (Figure 1.16). These compounds are highly corrosive and toxic and should be removed in the refinery. Nickel, vanadium, and copper can also severely affect the activities of catalysts and result in lower quality products. Organometallic compounds tend to concentrate in heavy, or residual fractions of crude oils.


Knowledge Check
What is the principal type of air pollution caused by the emission of sulfur oxides into the atmosphere?
Paraffins
Paraffins
Paraffins are also called alkanes and have the general formula of CnH2n+2, where n is the number of carbon atoms in a given molecule. Paraffins are divided into two groups of normal and isoparaffins. Normal paraffins or normal alkanes are simply written as n-paraffins or n-alkanes, and they are open, straight-chain saturated hydrocarbons. The second group of paraffins is called isoparaffins, which are branched-type hydrocarbons, and they begin with isobutane (also called methylpropane), which has the same closed formula as n-butane (C4H10). Compounds of different structures with the same closed formula are called isomers (Figure 1.9). For example, the open formula for n-butane, n-C4, can be shown as CH3-CH2-CH2-CH3, based on the quadrivalency of the carbon atom, and for simplicity, only the carbon-carbon bonds are drawn and most C-H bonds are omitted, as shown in Figure 1.7 and 1.8 on the previous page. Paraffins are the largest series of hydrocarbons found in petroleum and beginning with the simplest compound, methane.
Under standard conditions of temperature and pressure (STP), the first four members of the alkane series (methane, ethane, propane, and butane) are in gaseous form, and compounds starting from C5H12 (pentane) to n-heptadecane (C17H36) are liquids (constituting large fractions of hydrocarbons found in liquid fuels (e.g., gasoline, jet fuel, and diesel fuel), whereas n-octadecane (C18H38) or heavier compounds exist in isolation as wax-like solids at STP. These heavier paraffins are soluble in lighter paraffins or other hydrocarbons and can be found in diesel fuel and fuel oils. Paraffins from C1 to C40 usually appear in crude oil (heavier alkanes in liquid solution, not as solid particles) and represent up to 20% of crude by volume.
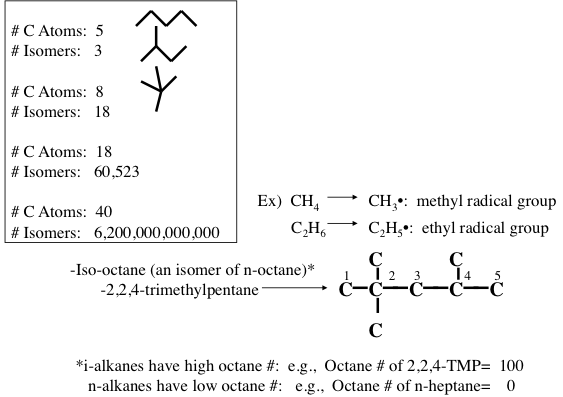
Figure 1.10 shows the statistically possible number of isomers of paraffins that increase exponentially with carbon number, starting with just one isomer for butane, reaching approximately 60,000 for C18 paraffins. Note that the branching in hydrocarbons causes significant changes in physical properties (e.g., boiling point and density, Figure 1.11) and chemical behavior (e.g., octane number, Figure 1.10) of paraffins with the same carbon number. Note in Figure 1.10 that the removal of an H atom from alkanes generates free radicals (reactive species containing unpaired electrons) that are called alkyl species (e.g., methyl formed from methane and ethyl formed from ethane by removing a hydrogen atom) also a radical with an unpaired electron. Also note the nomenclature using alkyl groups to specifically name isoalkanes (e.g., 2,2,4-trimethylpentane to designate a specific iso-octane).

Naphthenes or cycloalkanes are rings or cyclic saturated hydrocarbons with a general formula of CnH2n5H10), cyclohexane (C6H12), and their derivatives such as n-alkylcyclopentanes are normally found in crude oils.
