Lesson 3: Overall Refinery Flow
Lesson 3 Overview
Overview
Video: FSC 432 Lesson 3 (2:16)
Overview
Selected properties of crude oil provide information on its quality and the conditions for the optimum operation of a petroleum refinery for processing the crude oil to produce the desired fuels. Readily measurable physical properties of crude oil (such as density, boiling point, and viscosity) not only help in predicting the physical behavior of crude oil during refinery but also give insight into the chemical composition of the oil. Therefore, physical properties can be used in developing characterization factors that relate to the chemical behavior of crude oil and the characteristics of the resulting refinery products. In addition to using characterization factors, crude oils are classified using ternary diagrams reflecting the hydrocarbon composition in terms of paraffins, naphthenes, and aromatics.
As introduced in Lesson 1, petroleum refining integrates four types of processes: separation, conversion, finishing, and supporting processes. This lesson involves a quick walk through a simple refinery in the U.S. to see what happens to a barrel of crude oil, and to provide more detail on how different processes are sequenced for optimum operation. The simple animation below shows a simplified diagram of processing network to maximize gasoline yield and produce the other distillate fuels (jet fuel, diesel fuel, and fuel oil) in high yield.
The first sequence of processes in a refinery makes use of physical separation to wash the salt out and to fractionate the desalted crude into different boiling ranges in a distillation column. Following the distillation, these fractions are subjected to further separation processes, such as those in Light Ends Unit (LEU) dewaxing and deasphalting units; to finishing processes, such as hydrotreatment; and to conversion processes, such as catalytic cracking, hydrocracking, visbreaking, and delayed coking. As shown in the animation below, the final products from these processes include Liquefied Petroleum Gas (LPG), lubricating oil base stock, asphalt, jet fuel and diesel fuel, gasoline, fuel oil, and petroleum coke. Some fractions from LEU are sent to finishing processes (blending and hydrotreatment) and further to a conversion process (reforming) to produce additional gasoline. Light products from catalytic cracking are subjected to further conversion in the alkylation process to produce more gasoline. Finally, supporting processes, hydrogen production and sulfur recovery, help remove the major heteroatom contaminant, sulfur, from the petroleum fuels through hydrotreatment [1].
This refinery scheme is typical in U.S. refineries where the premium product is gasoline, as one could tell from the number of processes that lead to gasoline as the major product. The gasoline streams from different processes are blended in sophisticated linear and non-linear programming schemes to produce the three grades of gasoline sold in the U.S., regular, intermediate, and premium grades defined in reference to octane number. Elsewhere in the world, there is more emphasis on producing diesel fuel rather than gasoline, since the transportation systems are not as heavily dependent on gasoline-powered passenger vehicles. Diesel fuel is preferred for mass transport options (e.g., buses and trains), as diesel engines (with compression-ignition) can deliver more power than spark-ignition gasoline engines.
In the following sections, each major process group in a refinery network will be introduced in sequence. We will discuss how they fit in the “industrial ecology” of petroleum refining for the overall economic goal of maximizing profit in the prevailing markets for crude oil and the refined petroleum products. The video below presents a flow diagram integrating the four types of processes in a petroleum refinery.
Video: FSC 432 Simple Refinery Flow (4:36)
Learning Outcomes
By the end of this lesson, you should be able to:
- illustrate the refinery processes with examples for each category of processes;
- distinguish and evaluate the functions of different refinery processes to control refinery product yield and composition;
- evaluate the principles behind the major refinery processes and examine the products from each process from Distillation to Hydrocracking;
- formulate strategies for upgrading heavy oil.
What is due for Lesson 3?
This lesson will take us one week to complete. Please refer to the Course Syllabus for specific time frames and due dates. Specific directions for the assignments below can be found on the Assignments page within this lesson.
| Readings: | J. H. Gary, G. E. Handwerk, & Mark J. Kaiser, Chapter 1, pp. 32-36; Chapter 2, pp. 41-55 and the course material from this site |
|---|---|
| Assignments: | Exercise 2: Using ternary classification to characterize crude oil blends |
Questions?
If you have any questions, please post them to our Help Discussion (not email), located in Canvas. I will check that discussion forum daily to respond. While you are there, feel free to post your own responses if you, too, are able to help out a classmate.
Desalting and Distillation
Desalting and Distillation
Although distillation is usually known as the first process in petroleum refineries, in many cases, desalting should take place before distillation (Figure 3.1). Salt dissolved in water (brine) enters the crude stream as a contaminant during the production or transportation of oil to refineries. If salt is not removed from crude oil, serious damage can result, especially in the heater tubes, due to corrosion caused by the presence of Cl. Salt in crude oil also causes reduction in heat transfer rates in heat exchangers and furnaces.
The three stages of desalting are:
- adding dilution water to crude;
- mixing dilution water with crude by a mixer;
- dehydration of crude in a settling tank to separate crude and sediment and water (S&W).
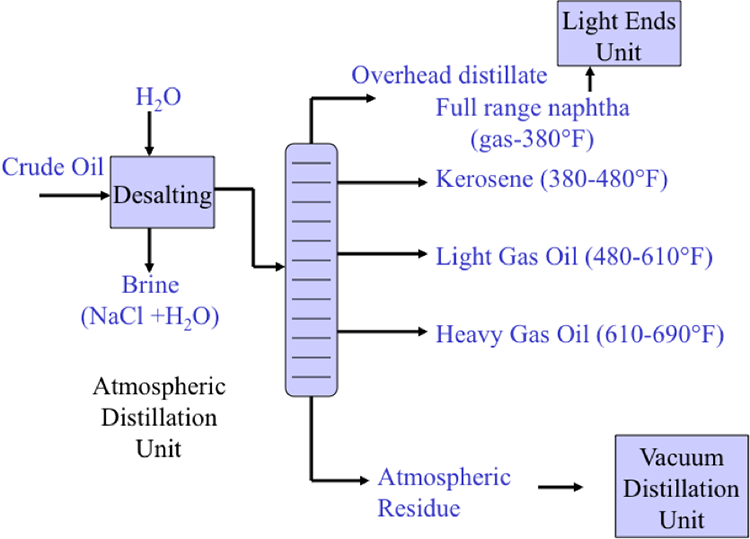

Desalting can be performed in a single-stage or two-stage units. The amount of water wash and the temperature of the mixing process depends mainly on the crude API gravity [2].
Distillation separates hydrocarbon compounds into distillate fractions based on their boiling points or volatility. More volatile compounds (with low boiling points) tend to vaporize more readily than heavy compounds, and this forms the basis of separation through distillation. In a distillation column, light components are removed from the top of the column, and the heavier part of the mixture appears in the bottom. For a crude that is a mixture of thousands of hydrocarbons, some very light compounds such as ethane and propane only appear in the top product, while extremely heavy and non-volatile compounds such as asphalts only appear in the bottom. Figure 2 shows a simple diagram of atmospheric and vacuum distillation units and the fractional separation of the crude oil into different boiling fractions with the indicated boiling ranges. The lightest compounds found in crude oil come out from the top of the distillation column (referred to as overhead distillate, or full-range naphtha) and are sent to the Light Ends Unit (LEU) for further separation into LPG and naphtha, as discussed later. The side streams separated in the atmospheric distillation column give fractions that include the “straight-run” products called kerosene, and light and heavy gas oils. The residue from the atmospheric distillation column generates two side streams, light and heavy vacuum gas oils, and vacuum residue from the bottom. All of these distillation products are subjected to subsequent processing to produce light and middle distillate fuels and non-fuel products, as described in the following sections, starting with LEU.
Light Ends Unit
Light Ends Unit
As shown in Figure 3.2, the Light Ends Unit consists of a sequence of distillation processes to separate the overhead distillate product from the atmospheric distillation column into five streams consisting of methane and ethane (C2 and lighter), to propane (C3), butane (C4), light naphtha, and heavy naphtha. The fraction C2 and lighter is used as fuel gas in the refinery to provide heat or generate steam. Propane and butane are sold as liquefied petroleum gas (LPG) after removing H2S. Light naphtha fraction that consists of C5 and C6 paraffins (pentane and hexane) is sent to the gasoline blending pool as straight-run gasoline, while the heavy naphtha fraction (rich in cycloalkanes, or naphthenes) is sent to a catalytic reforming process to produce gasoline with a high octane number.


Catalytic Reformer
Catalytic Reformer
Catalytic reforming converts low-octane straight run naphtha fractions (particularly heavy naphtha that is rich in naphthenes) into a high-octane, low-sulfur reformate, which is a major blending product for gasoline (Figure 3.3). The most valuable byproduct from catalytic reforming is hydrogen, which is needed in refineries with increasing demand for hydrotreating and hydrocracking processes. Most reforming catalysts contain platinum supported on alumina, and some may contain additional metals such as rhenium and tin in bi-, or tri-metallic catalyst formulations. Early reforming processes were called platforming in reference to reforming with a platinum catalyst. In most cases for catalytic reforming, the naphtha feedstock needs to be hydrotreated before reforming, to protect the platinum catalyst from poisoning by sulfur or nitrogen species. The principal reactions in catalytic reforming include dehydrogenation of naphthenes to aromatics (with significant quantity of hydrogen as byproduct) and cracking/isomerization of n-paraffins into i-paraffins. The principal product from catalytic reforming is called reformate, consisting of C4 to C10 hydrocarbons. Reformate has a high octane number because of high concentration of aromatic compounds (benzene, toluene, and xylene) produced from naphthenes. With the more stringent requirements on benzene and total aromatics limit in US and Europe (less than 1% benzene, 15% total aromatics), the amount of reformate that can be used in gasoline blending has been limited, but the function of catalytic reforming as the only internal source of hydrogen continues to be important for refineries.

Catalytic Hydrotreatment
Catalytic Hydrotreatment
As seen with the catalytic reforming in the previous section, catalytic hydrotreatment can be used as a pretreatment step to protect catalysts from crude oil contaminants such as heteroatom (S, N, O) compounds, as well as metals (mainly Ni, V). Hydrotreatment is also used as a major finishing process in a petroleum refinery. Shifting to the side stream products from the distillation column, kerosene and light gas oil fractions can be hydrotreated to remove the heteroatoms to produce the final products of jet fuel, and diesel fuel, as shown in Figure 3.4. Particularly strict sulfur limits are imposed on diesel fuels so that the particulate emissions from diesel engines can be reduced. In the U.S., the government regulations [3] require that highway and non-road locomotive and marine (NRLM) use diesel fuel that meets a maximum specification of 15 parts per million (ppm) sulfur by 2014, with a full compliance for highway use and non-road diesel fuel since December 2010. Note in Figure 5 that typical catalysts used for hydrotreating are Co and Mo compounds supported on alumina (Al2O3). Jet fuel consists of C10 to C15 hydrocarbons, and diesel fuel consists of C15 to C20 hydrocarbons. Analogous to octane number for gasoline, a performance parameter for diesel fuel is cetane number (n-C16H34, n-hexadecane) that measures, in contrast to octane number, the tendency (not resistance) of diesel fuel to ignite upon compression with air. As a side note, light gas oil fraction is not typically used in the U.S. for producing diesel fuel, but sent to catalytic cracking to make gasoline.

Conversion of Heavy Gas Oil
Conversion of Heavy Gas Oil
Moving down to the side streams of the distillation column, heavy gas oil constitutes the next fraction in line. Some generic conversion processes for the heavy distillates, such as heavy gas oil (consisting of C20 to C25 hydrocarbons), are shown in Figure 3.5. These processes, aimed at reducing the molecular size or the boiling point of gas oil compounds, involve thermal cracking or catalytic cracking. A mild thermal cracking process, called visbreaking, is applied to reduce the viscosity of the feedstock, and it is more frequently applied to residual fractions, such as vacuum distillation residue. A more severe thermal cracking of heavy gas oil can be used to produce LPG and ethylene and light and middle distillates from heavy gas oil. A highly aromatic byproduct from thermal cracking is called ethylene tar. Ethylene is an important petrochemical feedstock, while ethylene tar can be used as feedstock to produce carbon blacks. Catalytic cracking is more frequently used for conversion of heavy gas oil to gasoline.

A particular process of catalytic cracking, Fluid Catalytic Cracking, is almost exclusively used worldwide in heavy gas oil and light vacuum gas oil conversion. This process produces high octane gasoline primarily, with important byproducts, including LPG, light olefins and i-alkanes, light cycle oil (LCO), heavy cycle oil (HCO), and clarified slurry oil (also called decant oil), as shown in Figure 3.6. LCO is used in the U.S. to produce diesel oil by hydrocracking, and decant oil can be used as fuel oil, feedstock for carbon black manufacturing, and to produce a special type of petroleum coke called needle coke. Needle coke has a microstructure that makes it a good precursor to graphite electrodes that are used in electric-arc furnaces to recycle scrap iron and steel. The manufacturing of graphite electrodes, using a byproduct from FCC used to produce gasoline, is considered a principal interface between petroleum refining and the iron and steel industry.

Conversion and Processing of Vacuum Gas Oils
Conversion and Processing of Vacuum Gas Oils
Moving to the vacuum distillation column, the vacuum distillates, light vacuum gas oil (LVGO) and heavy vacuum gas oil (HVGO) can be processed by some advanced FCC processes. However, hydrocracking is more frequently used to convert LVGO and HVGO into light and middle distillates, using particular catalysts and hydrogen. Similar to LCO, the LVGO and HVGO fractions from vacuum distillation tend to be highly aromatic. Catalytic hydrocracking combines hydrogenation and cracking to handle feedstocks that are heavier than those that can be processed by FCC, because of excessive coke deposition on the catalyst in the absence of hydrogen. Middle distillates (e.g., kerosene and diesel fuel) are the principal products of hydrocracking. In addition to light and middle distillates, hydrocracking also produces light distillates and LPG, as shown in Figure 3.7.
HVGO can also be used as a feedstock to produce lubricating oil base stock, through a sequence of solvent extraction processes to remove aromatic hydrocarbons by furfural extraction, and to remove long-chain paraffins by dewaxing (Figure 3.8).


Processing and Conversion of Vacuum Distillation Residue
Processing and Conversion of Vacuum Distillation Residue
The heaviest and the most contaminated component of crude oil is the vacuum distillation residue (VDR), also referred to as the bottom-of-the-barrel. There are multiple processing paths to upgrade VDR into usable products. One process is called deasphalting, which removes the heaviest fraction of VDR as asphalt that is used mainly to pave roadways. The lighter fraction obtained in the deasphalting process, deasphalted oil (DAO), can be used as fuel oil after hydrotreatment (Figure 3.9).

Thermal processes, such as visbreaking and coking, also provide options for upgrading VDR, which is normally a solid at ambient temperature. As shown in Figure 3.10, the visbreaking operation involves mild thermal cracking, with the primary purpose of producing a relatively low grade fuel oil (with a much lower pour point than VDR) and byproducts such as middle and light (naphtha) distillates and LPG. The yield of these byproducts would normally not exceed 10%wt of VDR. As a general rule in refinery conversion processes, producing a lighter product with a higher H/C ratio (e.g., fuel oil, middle distillates, LPG) from a feedstock (e.g., VDR) would require the simultaneous formation of a heavier product (e.g., coke) with a lower H/C ratio than the feedstock. Clearly, this compensation is dictated by the hydrogen balance, or hydrogen distribution among the products. With no external hydrogen entering the conversion unit (as it would in hydrogenation, or hydrocracking reactions), making a product(s) with a higher H/C ratio than that of the feed would require making other product(s) with a lower H/C ratio than that in the feedstock. In the case of visbreaking, what enables the production of lighter (or lower viscosity) fuel oil and other products from VDR is the formation of small quantities of coke with an extremely low H/C ratio. Hence, the term disproportination describes this unequal distribution of C or H in the conversion products, or losing (rejecting) C in the coke that accumulates on reactor tubes and is periodically burned out to clean the reactor tubes.

As different from visbreaking, coking involves severe thermal cracking with intentional production of coke with a low H/C, so that lighter fuels can be obtained from VDR (by disproportionation), as can also be seen in Figure 3.10. The product coke obtained from VDR with relatively low heteroatom concepts, termed sponge coke, can be used in manufacturing carbon anodes that are used in electrolysis of alumina (Al2O3) to produce aluminum metal. This is another important interface between petroleum refining and metals industries, similar to the coke produced from decant oil used for making needle coke for manufacturing graphite electrodes to operate electric-arc furnaces, as mentioned before.
Paths for Upgrading Heavy Oil
Paths for Upgrading Heavy Oil
It should be clear from this quick tour of a refinery, that the most valuable products from a refinery include light distillates (gasoline) and middle distillates (jet fuel and diesel). These products are mostly paraffinic and contain relatively short paraffin chains, or small molecules, or, in other words, high H/C ratios. In this context, one could summarize the overall goal of petroleum refining as managing the H/C ratio of the products for the optimum distribution of hydrogen into products to maximize profits. Controlling the H/C ratio of the products would require either lowering the C content of the products (i.e., carbon rejection), or increasing the H content (i.e., hydrogen addition). The animation below depicts these two major paths for upgrading heavy oil (or crude oil) with some examples for each path. The processes, coking, solvent extraction (e.g., deasphalting), visbreaking, and catalytic cracking reject carbon in the coke (carbonaceous) product so that lighter products (with high H/C) ratios can be obtained in these processes. Carbon in the coke, or in the heavier product, is considered to have been rejected (and potentially lost) since the carbonaceous byproducts have much lower value in comparison to those of the lighter products. In contrast, hydrogen addition, as in the processes of hydrogenation and hydrocracking, enables the conversion of all the carbon present in heavy oil (or crude oil) to high value products without rejecting, or sacrificing, any. One might ask, then, why would any refinery carry out any carbon rejection process instead of hydrogen addition? A short answer to this question involves basic refinery economics; the hydrogen addition processes cost much more than carbon rejection processes, because producing hydrogen and the catalysts used in hydrogen addition processes are very expensive.
Video: FSC 432 Upgrading Heavy Oil (3:48)
Self-Check Questions
Self-Check Questions
Please take a few minutes to answer the following questions. When you are happy with your responses, click Check below.
Assignments
Assignment Reminder
Each week, you will have a number of assignments. This week's assignments are listed below with instructions on what to do. For due dates, please check your Syllabus.
Exercise 2
Exercise 2 Instructions
Exercise 2 is provided as a downloadable file in Canvas module Lesson 3. Please submit answers as a pdf to the Exercise 2 assignment in the Lesson 3 Module.
Please Note:
Scans of handwritten pages are not acceptable.
- A refinery has access to two different crude oil stocks A and B with the following compositions:
Table 3.1 Naphthenes % Aromatics % Crude A 30 65 Crude B 10 35 - What type of crude oils are A and B according to the ternary classification based on composition? 20 pts
- What type of crude would be obtained if A and B are blended in a proportion of A/B =3/2? 20 pts
- The refiners would like to maintain a weight ratio of 1/1, Crude A/Crude C in a ternary blend of the oils A, B, and C. What would be the minimum concentration of Crude B (% wt) in a ternary blend that could be classified as naphthenic oil? 60 pts
Table 3.2 aromatics naphthenes paraffins Crude A 70%wt 10%wt 20%wt Crude B 25%wt 60%wt 15%wt Crude C 15%wt 25%wt 60%wt
Instructions for Submitting Response:
Once you have a solution to the exercises, you will submit your answers as a PDF by uploading your file to be graded. The MS Word, or Excel files should be saved as a PDF before submitting the exercise. Please Note: Scans of handwritten pages are not acceptable.
Please follow the instructions below.
- Find the Exercise 2 assignment in the Lesson 3 Module.
- Make sure that your name is in the document title before uploading it to the correct assignment (i.e., Lesson3_Exercise2_Tom Smith).
Answers to Lesson 3 Exercise 2
Answers to Lesson 3 Exercise 2
Question 1
A refinery has access to two different crude oil stocks, A and B with the following compositions:
| Naphthenes % | Aromatics % | |
|---|---|---|
| Crude A | 30 | 65 |
| Crude B | 10 | 35 |
- What type of crude oils are A and B according to the ternary classification based on composition?
- What type of crude would be obtained if A and B are blended in a proportion of A/B =2/3?
Answer:
- What type of crude oils are A and B according to the ternary classification based on composition?
Aromatic-Naphthenic crude (Aromatics > 50% , Paraffins < 10 % )
Paraphinic crude ( P + N > 50 % , P >N , P > 40 % ) - What type of crude would be obtained if A and B are blended in a proportion of A/B =2/3?
In the blend with A/B = 2/3: A: 40%, B=60%
Binary Blend C: Naphthenes = (0.4)(30) + (0.6)(10) N= 18%
Aromatics = (0.4)(65) + (0.6)(35) = 30%, A=47%
Binary Blend C: Paraffinic-Naphthenic (A<50%, P<40, N<40%)
Question 2
The compositions of three crude oils available to a refinery are as follows:
Crude A: 60%wt paraffins, 20%wt naphthenes
Crude B: 50%wt aromatics, 30%wt paraffins
Crude C: 10%wt paraffins, 20%wt naphthenes
The refiners would like to maintain a weight ratio of 1/1, Crude A/Crude C in a ternary blend of the oils A, B, and C. What would be the minimum concentration of Crude B (% wt) in a ternary blend that could be classified as naphthenic oil?
Answer:
| aromatics | naphthenes | paraffins | |
|---|---|---|---|
| Crude A | 70%wt | 10%wt | 20%wt |
| Crude B | 25%wt | 60%wt | 15%wt |
| Crude C | 15%wt | 25%wt | 60%wt |
Set A+B+C = 100 and A=C
Naphtene balance:
0.1(100-B)/2 + 0.6B + 0.25(100-B)/2 > 40
0.42B >22.5
B > 54 (approximately)
B>54, Therefore, B must be greater than 54% to maintain a naphthenic crude blend.
Here is a similar exercise you may want to work on before looking at the answers given here, including a graphical solution using the ternary diagram.
Question 1
A refinery has access to two different crude oil stocks, A and B with the following compositions:
| Naphthenes % | Aromatics % | |
|---|---|---|
| Crude A | 10 | 60 |
| Crude B | 60 | 10 |
- What type of crude oils are A and B according to the ternary classification based on composition?
- What type of crude would be obtained if A and B are blended in a proportion of A/B =2/3?
Answer:
- What type of crude oils are A and B according to the ternary classification based on composition?
Aromatic-Intermediate crude (Aromatics > 50% , Paraffins > 10 % )
Naphthenic crude ( P + N > 50 % , N > P , N > 40 % ) - What type of crude would be obtained if A and B are blended in a proportion of A/B =2/3?
In the blend with A/B = 2/3: A: 40%, B=60%
Binary Blend C: Naphthenes = (0.4)(10) + (0.6)(60) = 40%
Aromatics = (0.4)(60) + (0.6)(10) = 30%, P=30%
Binary Blend C: Border-line between Paraffinic-Naphthenic (A<50%, P<40, N<40%) and Naphthenic ((P+N>50%, N>P, N>40%) crude oils.
You may solve the problem graphically using a ternary diagram, as described below.
Video: FSC 432 Blend Triangle (4:43)
Question 2
The compositions of three crude oils available to a refinery are as follows:
Crude A: 60%wt paraffins, 20%wt naphthenes
Crude B: 50%wt aromatics, 30%wt paraffins
Crude C: 10%wt paraffins, 20%wt naphthenes
The refiners would like to maintain a weight ratio of 1/1, Crude B/Crude C in a ternary blend of the oils A, B, and C. What would be the minimum concentration of Crude A (% wt) in a ternary blend that could be classified as paraffinic oil?
Answer:
| aromatics | naphthenes | paraffins | |
|---|---|---|---|
| Crude A | 20%wt | 20%wt | 60%wt |
| Crude B | 50%wt | 20%wt | 30%wt |
| Crude C | 70%wt | 20%wt | 10%wt |
Set A+B+C = 100g and B=C
Paraffin balance:
0.60A + 0.3(100-A)/2 + 0.1(100-A)/2 > 40
1.2A + 30 - 0 .3A + 10 - 0.1A >80
.8A > 40
A>50, Therefore, A must be greater than 50% to maintain a paraffinic crude blend.
Summary and Final Tasks
Petroleum refining may be considered as the most sophisticated scheme of integrated physical and chemical processes to meet the market demand for a number of fuels and materials in the economy. In addition to satisfying the performance specifications required by combustion engines, the composition of the produced fuels, such as gasoline, jet fuel, and diesel fuel, should be in compliance with environmental regulations. Considering that crude oil is a natural material that displays a wide range of variability in hydrocarbon composition and the distribution of heteroatom species, it is vital to practice an optimum sequence of the four types of processes that make up petroleum refining: separation, conversion, finishing, and support. These processes are integrated as an example of industrial ecology, such that every drop of crude oil ends up as a marketable product, including the contaminants such as sulfur. The U.S. refineries are configured to maximize the yield of gasoline (a light distillate) as the major product, along with jet fuel and diesel fuel (middle distillates). A number of processes produce different gasoline streams that are blended in sophisticated linear and non-linear programming schemes to produce the three grades of gasoline sold in the U.S. for profit. A quick walk through the network of refinery processes reveals the two general strategies that are in place to create the most value in the operation: carbon rejection and hydrogen addition. The balance between these two strategies hangs in the refinery economics and the markets for crude oil and refined petroleum products.
Learning Objectives
You should now be able to:
- illustrate the refinery processes with examples for each category of processes;
- distinguish and evaluate the functions of different refinery processes to control refinery product yield and composition;
- evaluate the principles behind the major refinery processes and examine the products from each process, from Distillation to Hydrocracking;
- formulate strategies for upgrading heavy oil.
Reminder - Complete all of the Lesson 3 tasks!
You have reached the end of Lesson 3! Double-check the to-do list below to make sure you have completed all of the activities listed there before you begin Lesson 4. Please refer to the Course Syllabus for specific time frames and due dates. Specific directions for the assignment below can be found within this lesson.
| Readings: | J. H. Gary, G. E. Handwerk, Mark J. Kaiser, Chapter 1, pp. 32-36; Chapter 2, pp. 41-55 and the course material from this site |
|---|---|
| Assignments: | Submit Exercise 2 as a PDF, or Excel file to the Exercise 2 assignment in the Lesson 3 Module. |
Questions?
If you have any questions, please post them to our Help Discussion (not email), located in Canvas. I will check that discussion forum daily to respond. While you are there, feel free to post your own responses if you, too, are able to help out a classmate.