Module 10: Uniformitarianism & the Age of the Earth
Welcome to Module 10
Welcome to Module 10: Uniformitarianism and the Age of the Earth
In Module 9, we learned how to read the rock record and write the history of the Earth, learning what happened and putting those events in order. These techniques, and the history they tell, were worked out by pioneering geologists mostly in the 1700s and 1800s. Those pioneers knew they were studying a very long history, but they couldn’t put precise numbers on exactly how long. It took until the second half of the 1900s for scientists to develop the knowledge and the sensitive instruments needed to learn how many years ago the events happened. The answer is given in this short video comparing time to distance on a US football field, and then the rest of this Module tells you a little about how the answer was discovered, with visits to Great Basin National Park and the Grand Canyon.
Video: 100 Yards of Geologic Time (3:06 minutes)
Click Here for Transcript of 100 Yards of Geologic Time Video
It is 4.6 billion years ago. You were on the goal line Beaver Stadium and you have to drive 100 yards to today where you're taking Geosciences 10. The earth is forming 4.6 billion years ago. Giant meteorites are streaming in. And when they hit the surface, they make great explosions and collisions that heat the planet so much that they evaporate the ocean. And the last one of those that was big enough to evaporate the upper part of the ocean that was warmed by the sun and given energy by the sun is about 3.8 billion years ago, which leaves you 83 yards to drive to get to the goal.
Beyond this, the continents are forming. They're no longer getting blasted. And so you start to see continents show up that the cores of the modern things and they're sitting out there very nicely. And they are formed so that you get an idea of what the world is going to be like by about 2.5 billion years ago, which is a mere 54 yards to get your touchdown.
There are bacteria in the ocean and the bacteria are committing acts of flatulence. They're putting oxygen up. The oxygen changes the composition of the atmosphere, it changes the oceans, and it eventually allows bigger critters to appear. And those bigger creators include shelly critters which suddenly make lots of interesting rocks, limestones. And so you start to get lots of shells showing up about 570 million years ago, which is a mere 12 yards to get the goal line. The shells are doing really well.
And then there's a really bad day. The ocean gets very warm from greenhouse and it belches out bad gases and most of the things alive die. And that happens about 225 million years ago at the end of the Paleozoic which is only five yards from the goal.
That clears up space so that you start to get dinosaurs. And as you know, dinosaurs were really big and they're sort of cute critters. And so you start getting dinosaurs in the Mesozoic. And here is a dinosaur if you would like one. and the dinosaurs are having a fine time and they're smiling a lot.
But there's another meteorite coming. And so the big meteorite comes screaming in and it kills the dinosaurs, and that changes the world a lot. And that happens about 65 million years ago which is only one and a half yards from the goal.
That makes room for mammals to show up, and so you start to see mammals such as this elephant that you're about to see here. This elephant happens to be running away from you. And the elephant has some big ears and a really curly tail.
And that comes up to recorded history. And recorded history, 6,000 years ago, just a little over the thickness of a sheet of paper.
And finally, to the culmination of creation to you, who are born about 1/200th of the thickness of a sheet of paper from the goal line today.
Imagine that the 100 yards of Penn State's Beaver Stadium, or any other football field, are like a timeline of all of Earth's history, and you're the star of the team, driving for glory. The planet formed on your goal line, half of the Earth's history had passed as your team marched across the 50-yard line, and now the coach personally sent you, the acme of creation, to carry the ball across the opposition's goal line of today for the winning score. If you have been carrying the ball for the whole 20 years of your life, how far did you run? (If you're not 20 years old, pretend.)
Congratulations—tomorrow's newspaper will report that you gained just a shade under 0.0002 inch, or a bit less than 1/200 of the thickness of a sheet of paper. The defense was vanquished by your onslaught, and instant replay officials were not needed to see that you broke the plane of the goal.
Written history goes back slightly less than 6000 years or so, barely the thickness of a sheet of paper on the 100 yards of Earth's "dark backward and abysm of time," as Shakespeare called it. Geologists often feel sorry for people who have restricted themselves to writings and skipped the rocks—those people may have seen the instant replay of the touchdown, but they missed the thrill of the game. So come along and see what happened before you carried the ball for those last two ten-thousandths of an inch!
Learning Objectives
- Understand that geologists learn ages of events in many ways, including counting annual layers in deposits, calculating backward from rates at which observed processes occur, and using many different radioactive-decay techniques.
- Explain how the results of these dating techniques agree with written histories as far back as writing goes, and agree with each other in demonstrating a vastly longer geologic history.
- Remember that science does not claim to be the ultimate Truth, but recognize that the science underlying age dating is very strong and that within science there is no “other side” that conflicts with the results here.
What to do for Module 10?
You will have one week to complete Module 10. See the course calendar for specific due dates.
- Take the RockOn #10 Quiz
- Take the StudentsSpeak #10 Survey
- Submit Exercise #5
- Begin working on Exercise #6
Questions?
If you have any questions, send an email via Canvas, to ALL the Teachers and TAs. To do this, add each teacher individually in the “To” line of your email. By adding all the teachers, the TAs will be included. Failure to email ALL the teachers may result in a delayed or missed response. For detailed directions on how to do this, see How to send an email in GEOSC 10 in the Important Information module.

This course is offered as part of the Repository of Open and Affordable Materials at Penn State. You are welcome to use and reuse materials that appear on this site (other than those copyrighted by others) subject to the licensing agreement linked to the bottom of this and every page. Students registered for this Penn State course gain access to assignments and instructor feedback and earn academic credit. Information about registering for this course is available from the Office of the University Registrar.
Main Topics: Module 10
Overview of the main topics you will encounter in Module 10
We visit Grand Canyon National Park, Arizona in this module, so before getting to the material that is likely to be on a quiz, we’ll start with some important thoughts from President Theodore Roosevelt on the value of saving our national treasures, from his speech at the Canyon on May 6, 1903. President Roosevelt went on to protect the Canyon, first as a Game Preserve and then as a National Monument, and it was made a National Park in 2019 under President Wilson.
Leave it as it is. You cannot improve on it. The ages have been at work on it, and man can only mar it. What you can do is keep it for your children, your children’s children, and for all who come after you, as one of the great sights which every American...should see.
We have gotten past the stage, my fellow-citizens, when we are to be pardoned if we treat any part of our country as something to be skinned for two or three years for the use of the present generation, whether it is the forest, the water, the scenery. Whatever it is, handle it so that your children’s children will get the benefit of it.
Parsley, Sage, Rosemary and “Time”
- In Module 9, we did “relative time”—which came first?
- Now we spice it up with “absolute time”—how many years?
- Count annual layers, for accurate estimates, for “short times” (less than about 100,000 years);
- Calculate from recent rates and reconstructed effects, for less-accurate but still reliable and useful estimates, for short and long times (uniformitarian approach);
- Use radiometric (radioactive) techniques, for accurate estimates, for short and long times.
Annual layers
- Overlapping tree rings, to more than 12,000 years;
- Special-lake sediments, to more than 45,000 years;
- Ice-core layers, to more than 100,000 years;
- MANY checks, including:
- reproducibility of counting;
- agreement with historical records (chemically fingerprinted fallout of historically dated volcanic eruptions, etc.);
- consistency between ice, lake, and trees dates for ages of abrupt climate changes;
- agreement with radiometric and uniformitarian ages.
- MANY checks, including:
Old as the Hills
- Annual-layer records from geologically young materials (from ice sheets, trees, and lake sediments that have not turned to stone yet, on top of rocks) extend back much older than written history;
- Data are clear that Earth looks much older than written history;
- Most religions agree;
Rocks you see while climbing out of the Grand Canyon
- Metamorphosed old mountain range at the bottom;
- Eroded surface (unconformity), then two miles of sediments;
- Tipped by faulting, then another unconformity, then another mile of sediments with several unconformities within;
- Rocks are familiar types, with animal tracks, mud cracks, etc., at many different levels, and changes in fossil types from layer to layer going upward;
- North Rim rocks then slant down under younger rocks at Zion, which are under Bryce rocks, which are under younger rocks, which are under prehistoric archaeological sites…
- Roughly 100 million years to deposit sediments, plus time for old metamorphics, plus erosion…
Radiometric Dating
- Half of parent atoms decay to offspring in one half-life (easy to measure; don’t need to wait for a half-life to pass, just for a measurable change);
- Half-life fixed by the same physics that makes the sun shine and keeps us alive;
- Measured parent:offspring ratio today plus measured half-life give the age of sample;
- Requires a little care and attention;
- Agrees with written records, layer counts, uniformitarian calculations, other radiometric techniques, and more.
Radiometric Dating Example
- Potassium-40 parent included in solidified lava flows, but gaseous argon-40 offspring escapes before liquid lava solidifies;
- After lava solidifies, the additional argon-40 produced from decay of potassium-40 is trapped;
- 1.3-billion-year half-life;
- If you start with 400 parents, after one half-life (1.3 billion years) average 200 parents left (and 200 offspring), after second half-life (total 2.6 billion years) average 100 parents left (and 300 offspring), after third half-life (total 3.9 billion years) average 50 parents left (and 350 offspring), …
0.0002 Inches and a Cloud of Dust
- Oldest rocks are about 4 billion years old; Earth bombarded by meteorites and mostly melted before that;
- Meteorites formed with Earth; they are about 4.6 billion years old, the same age estimated from radioactive dates of the Earth;
- If 4.6 billion years is the 100-yard length of a football field, all written history is about the thickness of a sheet of paper, and a 20-year-old person has lived through 0.0002 inches.
Great Basin National Park
Great Basin National Park
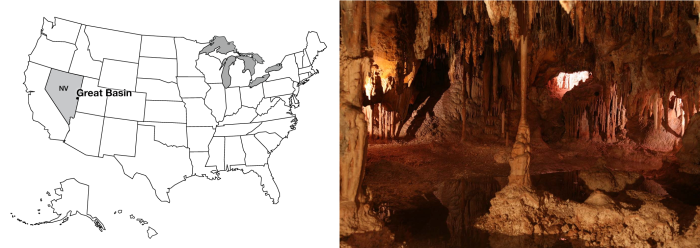
Out in eastern Nevada, a long way from almost any city, is Great Basin National Park. The jewel of Great Basin is Lehman Caves, one of the most "decorated" caves known, with a wide range of odd cave formations (stalactites and stalagmites, but lots more, too). (Note that the name is plural—Lehman Caves—but it is just one cave. We’re not sure why.) Lehman Caves is dissolved into marble (metamorphosed limestone) on the side of Wheeler Peak, which rises to more than 13,000 feet (almost 4000 m), and which was glaciated during the ice age; only a very small glacier remains in the cirque (about 2 acres). Great Basin is one of the less-visited national parks, with yearly attendance not too much over 100,000 visitors, so you can find a lot of solitude and wonder in this beautiful place.
Far up on Wheeler Peak, Great Basin bristlecone pines are living. These gnarled, straggly trees grow slowly in high, cold places, whereas bristlecone pines growing in warmer, moister, lower-elevation sites live faster and die younger. In part because of this slow growth, the high-altitude trees can be very old. The oldest known living bristlecone pine is more than 4,600 years old, in the White Mountains of California. The oldest tree known so far was cut on Wheeler Peak in 1964, when the land was still administered by the U.S. Forest Service, as part of a study to learn more about the growth and behavior of the trees. Now known as Prometheus, that tree was 4,950 years old when cut. That one old-looking tree was not notably different from many others in the large grove. Because it is so unlikely that the first such tree cut on Wheeler Peak out of the many there would happen to be the oldest tree on Earth, it is likely that there are older trees out there that have not been sampled yet.
Take a Tour of Great Basin National Park and Lehman Caves
Want to see more?
Visit the Great Basin National Park website. While you are not required to review this, you may find it interesting and possibly even helpful in preparing for the quiz!
Dating with Tree Rings and Other Annual Layers
Dating with Tree Rings and Other Annual Layers
Video: Dating with Annual Layers (3:39)
Trees make annual layers, and some sedimentary deposits also have annual layers. The longest annual records extend much older than written histories, although they capture only a very small part of Earth’s long history. In this Module, we start with annual layers and then continue to look at other ways to learn the ages of events in Earth’s history. If you like video versions, here’s a short intro.
Here's a little more about annual layers that we can use for dating events. Shown here is a tree sample that grew on Mount Saint Helens, and those really skinny rings were formed right after an eruption in the year 1480 that dumped ash on the tree and made it grow poorly. Many tree ring studies use bristlecone pines. These are Cedar Breaks National Monument in Bryce Canyon National Park. Bristlecone pines can live a long time. They're really tough. They hang on. This beautiful picture of a bristlecone pine was taken by Penn State alum and Professor John Fegyveresi, up in the white mountains of California. Bristolcone Pines can live at least almost 5,000 years, and the longest record of living and dead overlapping Bristolcone Pines goes to 8,800 years. Truly amazing. How you do it, you can find living wood, you can find dead wood nearby, you can find wood in archeological sites, such as this sample that I just highlighted in Mesa Verde. These are some more Mesa Verde samples. The left one in the lower right were in place in the Long House. The one in the upper right is in the museum, but it was taken from balcony house.
You use them like this. Find the living wood, find the dead wood, find the archeological wood, match up the pattern of thick and thin rings, and make a record which is longer than the life of one tree. You can also get annual layers in cave formations. All of these are different ways to look at cave formations or speleothems. The upper left is using an ultraviolet light to make the layer stand out more easily. The one in the upper right is under a microscope, and it's just shining light off of the cave formation and looking at the layers. Lower left is a thin slice has been cut, and the light is being shown through it, so you can see the layers. And lower right there are chemical measurements along a along speleothem across the layers so you can see the variations in the chemistry. You can find annual layers in corals, and on top you see the layers very nicely with dates appended to them there. It came, in this case, from a brain coral like the one down below. You can also find annual layers in the sediments of some lakes, such as this one from Germany or this one from Switzerland.
Now, when you use annual layers to date an abrupt climate change or the fallout of a volcano, the trees and the lakes and the cave formations and so on agree with each other. They agree with written history. They agree with radioactive dating. The longest annual records now go to more than 12,000 years in the trees, more than 45,000 years in the lakes, and more than 100,000 years in the ice caves.
In a seasonal environment, a tree reliably produces a visible growth ring each year. The reasons for this behavior are well-understood, and the annual nature of the rings has been checked many, many times. Rarely, there is a problem (a piece of a ring may be missing if the tree was damaged, perhaps by a fire or a burrowing beetle, and a late frost or other odd event may make a ring look strange), but tree-ring daters (dendrochronologists) have learned to recognize these events. In general, tree-ring dating can be practiced with no errors. Many, many tests have been conducted to confirm that this works, that the results match historical records, etc. Most such sampling is done using narrow coring devices, and does not harm the trees.
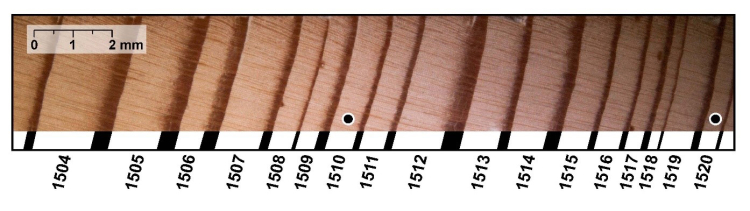
In studying tree rings, one sees that the width is not the same from year to year. Thick rings grow during “good” years, and thin rings during bad years. This allows tree rings to be used to reconstruct past climates. In a dry area, a good year is a wet one, so tree rings can be used to find out how much rain fell in the past. In a cold area, a good year is a warm one, so the tree rings function as thermometers.
For our purposes here, the pattern of good and bad years (fat and thin rings) is important for dating. On Wheeler Peak, and in the White Mountains and elsewhere, dead trees occur near the living bristlecones. Some of these dead trees sprouted before the living ones and overlapped in age with the still-living trees. Other dead trees can be found in archaeological sites or buried in sediments. A tree-ring specialist can start by dating the good and bad years using living trees. The specialist can then find the same pattern of thick and thin rings in overlapping years of the dead tree, and so use the dead tree to extend the record back to when the dead tree first sprouted (see the figure below). By overlapping a few long-lived trees, or many short-lived trees, very long chronologies can be generated.
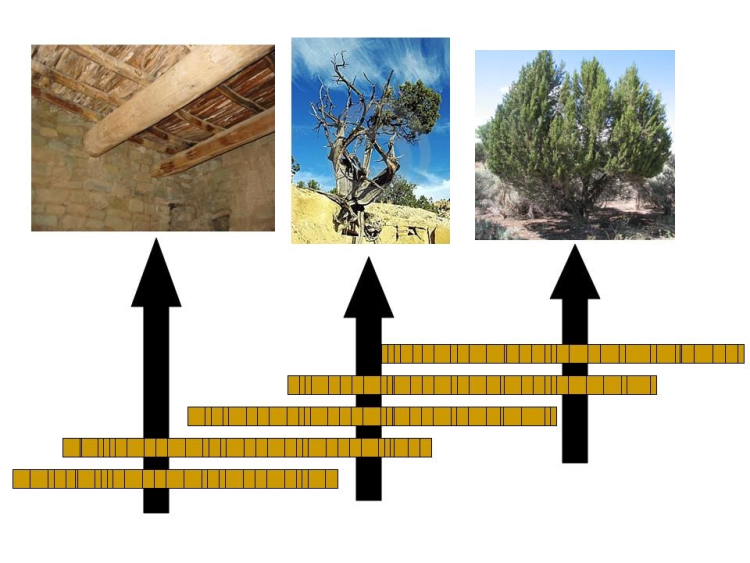
So How Many Layers?
Such techniques are used to date archaeological sites, including those of the Ancestral Puebloan peoples (also sometimes called the Anasazi; at Mesa Verde and several other national parks). For example, the Cornell Tree-Ring Laboratory, long directed by the great Professor Peter Ian Kuniholm and now being carried forward by a new generation, has for decades been doing amazing work using tree rings to understand classical history in the Aegean region, the Middle East, and elsewhere, confirming, refining, and extending historical accounts. The beautiful agreement between tree-ring and historical accounts as far back as the oldest reliable written records confirms the accuracy of the techniques.
But, the tree-ring records extend well beyond reliable written histories. The longest tree-ring record in the U.S. Southwest is now more than 8000 years. The longest record anywhere in the world is from tree trunks buried along rivers in north Germany, and extends to 12,429 years—before that, closer to the heart of the ice age, conditions were too cold for trees in that region of Germany, including times when the area was under massive ice-age glaciers. Because most trees live for “only” centuries rather than millennia, such records (and a few other really long ones, such as a 7,272-year record that was completed in 1984 from oak logs buried in Irish bogs) represent immense investments of time and effort, and people have devoted whole careers to assembling these outstanding records. Notice that there is a lot of older wood, some of it much older, including the fossil trees at Yellowstone, in the Petrified Forest, and elsewhere. The more than 12,000 years in Germany are the longest continuous record reaching the present, but surely do not come anywhere close to including the whole history of trees.
Other Annually Layered Deposits
Several other types of annually layered deposits exist. For example, some lakes in cold regions freeze every winter. When the lake is thawed in the summer, sand and gravel are washed in by streams. When the lake and its surroundings freeze, the streams slow or stop, and the only sediment settling to the lake bottom is the very fine silt and clay particles that were washed in during the summer but require months to fall. A coarse layer capped by a fine layer forms each year. Such a yearly coarse-fine layer pair is called a varve. Many such varved lakes have been studied, and found to contain thousands of years to more than 14,000 years. Many of these lakes occur in glacier-carved basins, and so their records extend only back to the time when the glacier ice melted.

Lake Suigetsu, in Japan, has a spring bloom of diatoms—algae with silica "shells"—that make a light-colored layer, alternating with darker mud washed into the lake during the rest of the year. More than 45,000 annual layers have been counted in that lake, although some interpolations were needed in a few places in the cores.
Note that most lakes lack annual layers. If there is a lot of oxygen in the deep waters, worms will thrive in the mud beneath, and their burrows may disturb the layers. If the lake is shallow, waves may disturb the deep muds. But enough lakes exist with annual layers to be useful. And, simply seeing layers doesn’t prove they are annual; lots of tests have to be done, some of which we describe below when we discuss annually layered ice cores.
Cave formations often have annual layers. And, a few other types of sediments, including certain corals, can have annual layers. Again, a lot of work goes into showing that the layers are annual, and into interpreting them accurately.
Dating with Ice Sheets
Dating with Ice Sheets

This image is of Kurt Cuffey, a Penn State student at the time, studying an ice core from GISP2, central Greenland, in the undersnow laboratory constructed for the project. Image taken by R. B. Alley © Penn State is licensed under CC BY-NC-SA 4.0
A difficulty in lakes—and other archives such as annually layered stalagmites in caves—is that an annual layer must be thick enough to be recognized, but a lake or a cave will fill up quickly if layers are thick, so the records cannot be extremely long.
Longer records are possible from the two-mile-thick ice sheets. Dr. Alley has been very active in this work, and Dr. Anandakrishnan has contributed in important ways. In central parts of the ice sheets, the temperature almost never rises high enough to melt any snow and ice. However, summer snow and winter snow look different because the sun shines on the snow in the summer, “cooking” the snow and changing its structure, but the sun does not shine on the winter snow, which is buried by new storms before the summer comes. You can count many, many layers by looking at an ice core, and Dr. Alley has done so, working especially on one core called GISP2, which was drilled just west of the summit of the Greenland ice sheet during the years 1989-1993.
Video: Ice Layers (5:04)
Here is a video showing how the GISP2 ice core was collected and analyzed, and then a written description with more information.
We're going to go north to learn about annual layers in ice cores from Greenland. I've labeled this a Good Day in Greenland. We had been there for a month, so those newspapers were actually not very current, but they were useful for some things. And so, this is a good day. This, you can see, is a bad day in Greenland. We were working up there all summer for many years. This is our fourth of July party. After a month or so up there, you'd want to rinse out your underwear. You can see the icicles on my freeze-dried laundry. We sometimes had really big storms, and after a storm, the snow surface might look like this. It would have a lot of snow drifts, but it's sort of smooth at the small scale.
And then we'd have these beautiful sunny days that almost but not quite got up to freezing. Then at night, this is the midnight sun, you would get fogs and they would grow frost on everything. Very spectacular frost. On the volleyball net, there next to my lens cap, where we would go out skiing for exercise. There's frost on everything. If I take my pocket knife and I slice out a little piece of snow and bring it up where you can see it, the wind-packed snow from the storm is down an inch or two, a couple of centimeters. The very top has actually sublimated. It was heated by the sun, the vapor went up in the air, and then it came down to make the frost. So you get this low density, coarse-grained layer on top of the snow.
Now, what are we going to do? There's Joan Fitzpatrick of the United States Geological Survey wearing the flannel shirt. That's me without a shirt. We're digging two holes. We're going to put a lid over one of them, the one that Joan and I are in, and let the sun shine through the wall from the other one. Here's me going down into a pit, and this is the wall. This happens to be only about 6 inches of it. Snow is blue more or less the same reason as that water is blue, the red is absorbed a little bit. We're looking at some of those low density layers that form during the summer when the sun is intense. If we back up and look at the whole wall. This was about 6 feet deep, which is as deep as we could dig easily.On top is the snow that accumulated during the previous winter, and then the previous summer, and another winter, and another summer, and another winter, and another summer, and then it's a little too deep for us to dig deeper. We did a huge amount of work to demonstrate that these really are winter, summer, winter, summer, winter, summer.
Now, we're going to drill an ice core with the big drill. Here's Katherine from Alaska on the big drill. Take the core, put it down into our Under Snow Laboratory. Here's Bill from New Hampshire down there. Wanda Kapsner, who was a Penn State student at the time, and went on to be a teacher and to run a winery, and she's studying a thin section of an ice core. Here are a bunch of cores. The 1547 there means 1,547 meters down. That's almost a mile where the ice was about 10,000 years old. Here's Kurt Cuffey, who was an undergrad then and became a famous professor, and he's studying an ice core. This is what he's looking at. This one is from 1411 meters down or about 8,400 years old. And there's a winter. The summers look dark now.
Those low density layers gave big crystals and big bubbles, which in this transmitted did light on our light table look dark. So the summer looks dark, and there's a winter, and there's a summer, and there's a winter. Again, huge amount of effort to test that this really is accurate, and it is. And we were able to count more than 100,000 annual layers in this core. So that's a little bit of how we get an annually resolved record from an ice core.
To verify that the layers are annual, several things were done. First, one person (Dr. Alley) looked at the core, waited a while, and then looked at it again to see that the counting is reproducible (without cheating by looking at the first count while making the second one). Then, several other people counted the layers visible in the core (without cheating by finding out what Dr. Alley had gotten), just to make sure they agreed.
There are many annual indicators in ice cores, probably more than a dozen. For example, the isotopic composition of the ice is a thermometer that records summer and winter. And sunshine makes hydrogen peroxide in the air in the summer when the sun shines, and the peroxide falls on the ice quickly, but there is almost no peroxide made and deposited in the dark winter. So annual layers have been counted using several different indicators, and they agree closely.
This is still not good enough. When a large volcano erupts, it throws ash and sulfuric acid into the stratosphere. These spread around the Earth. The bigger pieces of ash fall out quickly, often in days or less, while the sulfuric acid may take one to a few years to fall (and, until it falls, affects the climate by blocking a little of the sunlight). You can use electrical or chemical techniques to find the layers of volcanic fallout in ice cores. The key sections can then be cut out, melted, and filtered, and any volcanic ash that is found can be analyzed chemically and compared to that from known volcanic eruptions. So, if you count back to the year 1783 in a Greenland ice core, you are in the year of the great Icelandic fissure eruption of Laki, which spread dry fogs across Europe and is well recorded in histories—Ben Franklin commented on the fogs in Paris while he was ambassador there for the fledgling United States. In fact, ash of the composition of Laki occurs in Greenland ice cores at the level dated 1783 by layer counting—the layer counting is right (or very close—some counts missed by a year or two initially). Similarly, ash from many other historical volcanoes has been found, back as far as historically dated volcanoes are known.

Comparison of counts of strata by one person at different times, by different people, and by different methods, and comparison to volcanic fallout, yielded almost the same answers, within about one year in one hundred (so one person may count 100 years, and another will count 99, or 100, or 101, but not 107 or 93 or some similarly large error).
There are a few more tests yet. There were very large and very rapid climatic changes at certain times in the past. Ice cores record the climatic conditions locally (how much snow accumulated and how cold it was), regionally (how much dust and sea salt and other things were blowing through the air to the ice from sources beyond the ice sheet), and globally (by trapping bubbles of air, which contain trace gases such as methane that are produced across much of the Earth’s surface and that changed in the atmosphere when the abrupt climate changes affected the sources of the greenhouse gases). Changes in all of these indicators occur at the same level in the ice cores, showing that the climate changes affected much of the Earth.
These changes left their “footprint” in the ice of Greenland, and the lakes of Switzerland and Poland, and the trees of Germany, etc. So, different groups can date such changes in the annually layered deposits of all of these different places. And, the dates agree closely. These events also have been dated radiometrically (we’ll cover this soon), and the dates also agree closely. One event, for example, was a short-lived return to cold conditions in the far north during the warming that ended the ice age, and is called the Younger Dryas. Close agreement as to its age is obtained from all of these different layered deposits and from radiometric ages—the Younger Dryas ended and warmer conditions returned to the far north about 11,500 years ago.
Thus far, the layers in the ice cores provide the longest reliable records. Over 100,000 layers have been counted. High accuracy was achieved younger than about 50,000 years, with somewhat lower reproducibility (maybe 10% or so, and with well-understood reasons for the lower accuracy) older than about 50,000 years. Still older ice exists, but those still-older layers in Greenland have been mixed up by ice flow and no longer give a reliable chronology. Thus, we have high confidence of more than about 100,000 years from the ice cores. (Really old ice in Antarctica, to 800,000 years or so, got less snowfall in a year than the height of a snowdrift, so annual layers are not preserved reliably, and other dating techniques must be used.)
Deep Time
Deep Time: Why Are We Emphasizing This?

Deduced from The Origin of Time, and continued to the beginning of the Emperour Vespasians Reign, and the totall Destruction and Abolition of the Temple and Common-wealth of the Jews.
Containing the HISTORIE of the OLD and NEW TESTAMENT, With that of the MACCHABEES. Also all the most Memorable Affairs of Asia and Egypt, And the Rise of the Empire of the Roman Caesars, under C. Julius, and Octavianus.
COLLECTED From all History, as well Sacred, as Prophane, and Methodically digested,
By the most Reverend JAMES USSHER, Arch Bishop of ARMAGH, and Primate of IRELAND. LONDON
Printed by E. Tyler, for J. Crook, at the Sign of the Ship in St. Pauls Church-yard, and for G, Bedell, at the Middle-Temple-Gate, in Fleet-Street. M.DC. LVIII.
One of the great results of geology has been the concept of “deep time.” The world was once believed in some cultures to be only as old as the oldest historical records. The Archbishop Ussher of Ireland, in the year 1664, declared that based on Biblical chronologies, the creation of the Earth dates from October 26, 4004 BC, Adam and Eve were driven out of the Garden of Eden on Monday, November 10 of that year, and Noah’s Ark landed on Mt. Ararat on Wednesday, May 5, 1491 BC. Other Biblical scholars obtained slightly different dates, but with broad agreement that the world was no older than the few thousand years that are documented in written histories.
Ussher’s date rested on a literal reading of the particular translation of the Bible he used, and on quite a number of questionable interpretations of the text—the Bible itself never gives an age for the Earth. Early geologists nonetheless struggled with the constraints provided by such chronological readings—how could all of geologic history fit into 6000 years? The early geologists ultimately reached the conclusion that the world looks MUCH older than 6000 years; either the world is older than this, or we have been deliberately fooled by some powerful being who crafted a young world to look old. As scientists, we work with the observable part of the world, and we have no way to detect a perfect fake, so we treat this as an old world. The geologic record speaks of “deep time,” billions of years, Shakespeare’s “Dark backward and abysm of time" (from The Tempest).
Most modern Biblical scholars have reached the same conclusion: the chronologies of Genesis do not give the precise age of the Earth, and are perfectly compatible with an old Earth. Most of the large Christian denominations, for example, have accepted an old Earth based on Biblical and on scientific interpretations. In 1996, the pope added the Catholic Church to the wide range of protestant denominations that accept an old Earth.
It remains that some denominations and people insist on what is often called a “literal” reading of the Bible. In addition, a few very vocal people continue to argue that the Earth looks young. Many more people hear all of this commotion and figure that maybe there is something wrong with the science, because “where there’s smoke, there’s fire.” Other people take it as an element of faith to disbelieve the scientific evidence, and even to accuse scientists of being bad people for opposing the young-Earth interpretations.
In this course, we go to some length to show you a small bit of the evidence that the Earth does not look young—it bears the marks of a deep and fascinating history. The annual-layer counts by themselves require an old Earth, because the tree rings, the lake sediments, and the ice cores all extend to older than the historical chronologies. The Irish oaks preserve rings from more than twice as many years as Archbishop Ussher of Ireland would have said were possible since Noah's flood, and many old trees that are still alive today sprouted before the date Archbishop Ussher gave for Noah’s flood with no sign of any damage, so his prediction was tested, and failed. Geologic and other scientific evidence from tree rings, lake sediments, ice cores, archaeological sites, and more match historical records well as far back as those historical records go; indeed, such science has been important in confirming the historical accuracy of some testable parts of religious texts. But as we shall see in the next sections, those annual layers and other “young” things are only the tip of a very old, very deep iceberg.
Please note that it is not the author’s intent to insult or belittle anyone’s beliefs here. Science, you may recall, has no way of verifying whether it has learned the Truth; it is a practical undertaking designed to discard ideas that fail, save the ones that don’t fail as provisional approximations of the truth, and push ahead. The hypothesis of an Earth that is no older, and looks no older, than historical records, leads to many predictions. Geologists began seriously testing those predictions in the 1700s, and found that those predictions were not supported, whereas predictions of an old-Earth hypothesis worked well—with very high confidence, the rocks look very old.
Consider two people, A and B. A has decided that belief in a literal interpretation of their favorite translation of the Bible is the most important thing in their life, as it controls the fate of their eternal soul and their relation with the most powerful being in the universe. Is it possible for A to look at the rocks, trees, ice and lakes, and find some way to explain those data in the context of that literal belief? The answer, obviously, is yes; many people do so, and some of them may be unhappy with us for what we wrote here. Next consider person B, who is working in an oil-company laboratory trying to improve dating of petroleum generation and migration. Which works best for B in making sense of the sedimentary record, A’s young-Earth interpretation or that of the geological profession? The answer is equally clear; A’s view is completely unhelpful, but geology works. Finally, ask whether A can be a geologist and use the old-Earth tools to find oil and minerals and clean water even while believing the Earth is young, or whether B can be a religious leader while doing geology, and the answers are yes; some people can hold a variety of ideas in mind at the same time. But recognize that the scientific evidence for an old Earth (and later, for evolution) is about as clear as science gets, and that the level of scientific disagreement on these issues is about as low as disagreement ever gets in science. Within the scientific community, there is no argument about whether the Earth really is older than historical records, just as there is no scientific argument about whether the Earth is roughly spherical. (Lively discussions clearly continue in the blogosphere and in other many non-scientific circles, but those discussions are at best rather weakly linked to the science.)
The Grand Canyon National Park
Grand Canyon National Park
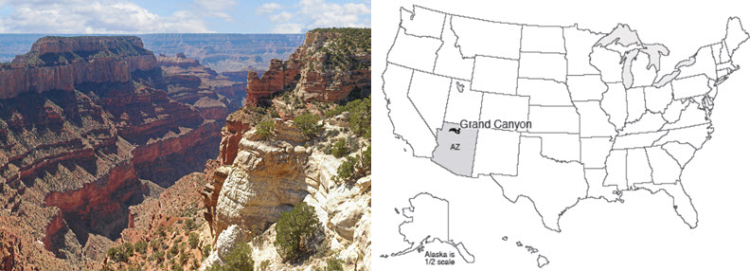
The Grand Canyon is a mile-deep, 18-mile wide, 277-mile long (1.6 km x 29 km x 446 km) gash in the Earth. The colorful spires, the rocky cliffs, the hidden pocket canyons, the pristine springs making lovely deposits, the roaring thunderstorms and arching rainbows are to many people the quintessence of the U.S. West. The Grand Canyon is neither the deepest nor the steepest canyon of the planet, but the Grand Canyon indeed is grand, and defines “canyon” for many people.
When the author, his sister Sharon, and his cousin Chuck were hiking the Bright Angel Trail from the North Rim into the canyon, a snake crossed the trail and slithered into some dry grass just at the trail edge. Chuck and I, in the lead, could see quite clearly that this snake ended in a “harmless” tail. Sharon, just behind, was not aware of the snake until it stuck its head out and rattled the grass just at her feet. Deciding that discretion was the better part of valor, and that if it rattles like a rattler it might actually be one, she made one mighty leap backward, landing in a cloud of dust on a switchback below.
Sharon almost certainly was not concerned with the rocks about her at that instant, but she had leaped backward through history. And what a history it is.
Want to see more?
Visit the Grand Canyon National Park website. While you are not required to review this, you may find it interesting and possibly even helpful in preparing for the quiz!
Hiking through History
Hiking Through History
The Penn State CAUSE class did what roughly 1% of the visitors to the Grand Canyon do, and hiked to the bottom and then back out. The trek down is rugged, often dusty, often hot, and safe only for well-prepared hikers. Many of the people who do make the hike report that it is the experience of a lifetime.
The rocks at the Grand Canyon are in order, with the oldest ones on the bottom, so in hiking back up from the river to the rim, we were hiking upward through history. The next section, The Longest Story, is a travelogue of the sites we saw on the way up. A lot of detail is provided, NOT to make you memorize it all, but to give you a small sample of the amazing things that geologists have learned, and how rich and varied the history of our planet really is.
So, lace on your boots, and let’s start the mile-high climb from the Colorado River to the rim of the Grand Canyon, watching the geology all the way.
The Longest Story
At the bottom, the river has cut the narrow, steep inner canyon through the Precambrian Vishnu and Brahma Schists. The older Vishnu has the appearance and chemical composition of metamorphosed sediments. The lava flows of the Brahma preserve the pillow structure of submarine eruptions, but the interbedded volcanic airfall material shows that at times the region was exposed as dry land. The total thickness of three miles of lava flows and interbedded layers, now standing almost on end although they initially were deposited almost horizontally, speaks of an important, long-lasting interval of deposition.
These oldest lava flows and sediments of the Grand Canyon have been "cooked," and are now of metamorphic types that form only in the hearts of mountain ranges at very high pressures and temperatures. During and after the metamorphism, melted rock (magma) squirted into cracks in these rocks, and then froze to form the pretty pink Zoroaster Granite. Yet this whole package of rocks was then brought back to the surface as the rocks of the mountains above them were eroded, with the erosion producing a very smooth, nearly horizontal plain on top of them, and weathering/soil formation causing changes that extend deep beneath that plain into these rocks.
The sea next advanced across this plain, first picking up and carrying and rolling pieces of the rocks and soils on the erosion surface to form a conglomerate, then giving way to sandstones, shales, and limestones that piled up to a thickness of two miles or so. (Such a great thickness does not mean that the sea was two miles deep; rather, in this case, the water stayed relatively shallow, but the warping of the crust by the drifting plates and other processes caused the sea floor to sink as the muds and other deposits piled up; recall that the Mississippi Delta is much more than 2 miles thick.) These rocks include mud cracks, ripple marks, casts of salt crystals that formed when the sea water evaporated in nearshore environments, and stromatolites, which are algal-mat deposits in which the algae trap mud, grow up through it, and trap more mud. All of these are similar to modern features, and indicate gradual accumulation (a layer, then drying for mud cracks, then more mud, then ripples from water flow, then drying for salt casts, and on and on and on).
Death-Valley-type pull-apart faulting then dropped and rotated these layers, so that they now slant (see the figure below). Long-term weathering and erosion then occurred, leading to a low, almost flat landscape broken by a few higher points where especially resistant rocks did not erode as rapidly. Again, deep weathering speaks of long exposure. In some places, the sediments were entirely removed down to the metamorphic rocks beneath, but in other places the sediments are preserved where they were dropped by faulting.
 View of the east end of the Grand Canyon, showing a little of the two miles of Precambrian sedimentary rocks, labeled “Supergroup”, which were dropped and tilted along Death Valley-type faults that are not visible here. Erosion for a long time produced the unconformity above, and then the sea returned to deposit the Tapeats Sandstone and rocks on top of it.
View of the east end of the Grand Canyon, showing a little of the two miles of Precambrian sedimentary rocks, labeled “Supergroup”, which were dropped and tilted along Death Valley-type faults that are not visible here. Erosion for a long time produced the unconformity above, and then the sea returned to deposit the Tapeats Sandstone and rocks on top of it.
The sea then returned, again reworking materials on the erosion surface to make a basal conglomerate, followed by beach sandstone, then offshore shale, and limestone from farther offshore. As the sea deepened and the beach moved towards the land, shale was deposited on sandstone, and then limestone was deposited on shale. These three layers, the Tapeats Sandstone with its thin basal conglomerate, the overlying, Bright Angel Shale, and then the Muav Limestone, form the slope that is known as the Tonto Plateau, and is so evident on the south side of the canyon. The rocks of the Tonto Plateau include fossils of marine animals such as trilobites, and even trilobite tracks. Again, all evidence is of deposition by processes just like those operating today, over long periods of time. A layer with a trilobite track must have been exposed long enough for a trilobite to crawl across it. The thousands and thousands of different layers in the rocks, with ripples and tracks and fossils, indicate long times.
Time then passed of which we have no record in the Grand Canyon, except that stream channels were carved on top of the Muav Limestone, indicating that the region was raised out of the sea and erosion was occurring. Fossils from two of the periods of the Paleozoic are missing, indicating that much time passed. When deposition resumed, the first rocks put down were limestones in the stream valleys, but another time gap sits on top of those in-the-channel rocks. The limestone in the channels, called the Temple Butte, includes coral and shellfish (brachiopod) fossils, and plates from armored fish.
Video: Grand Canyon Strata (8:22)
If you hike out of the Grand Canyon, these are the rocks you walk on. We don't want you to try to memorize everything here, but enjoy the hike and appreciate the history. It's truly amazing. We're going to start down here with the Vishnu and Brahma Shist, the Zoroaster granite. Here's a picture, two pictures of them down by the river. They started out as sediments and lava flows and ash that fell out of the air from volcanic eruptions. Then they were squeezed and heated in the heart of a mountain range, metamorphosed, and then squirted pink granite, the Zoroaster granite, into them and folded to make these beautiful things. But then they got all the way back to the surface so that they could be eroded to make the unconformity, the time gap there, before deposition of the sediments of the Grand Canyon Supergroup on top.
This is a fascinating bit of history here. The same erosion between these old shifts and granites and the Nices and the Grand Canyon Supergroup was cut by a fault, which dragged up the layers of the Grand Canyon Supergroup. But the fault quit moving before the next erosion surface and the deposition of the sediments on top of that, because that surface is not cut by the fault.
Now, we're going to go a little bit east in the canyon. We don't see the oldest rocks here, the Vishnu, is not here. The Vishnu is not here, it's below the river. But we can see the Grand Canyon Supergroup very well, and it is a big pile of sediments. This is that erosion surface on top of it. And how much sediment in the Supergroup? Measure down, walk along a layer, measure down, walk along a layer, measure down. Keep doing that. It's two miles of sediment piled on top of each other, deposited in shallow water next to a sea as the land was sinking and so more sediments could build up, drop down in death Valley-type faults. And they're just ordinary sediments. This, for example, is a little tiny piece of that. This is a stromatolyte. Algae would grow on a rock, mud washes in, the algae grow up through it, mud washes in. Each of those little layers you see in this picture is a day's worth of algae, and growing up and growing up and growing up.
Let's go back now. We've worked up through the Vishnu and Brahma and Zoroaster and the Grand Canyon Supergroup. Now we're going to go up and look at these layers, the Tonto Plateau, the Tapeats, and Bright Angel, and Muav, You can find a trilobite fossil in the Bright Angel. In the modern world, you know that it very often when water flows, it makes ripples. Well, these are old ripples in the Bright Angel Shale. Here's a picture I took of ripples viewed edge on in that tapete sandstone. Here's another picture of looking down on a layer in the tapete. A worm craw through this and left the track.
We'll go back to the canyon, and we're going to move up and see this wonderful story happening right there. It also tells us something about a story that happened out in the west edge of the canyon and gave us what's called the Surprise Canyon. In this picture, we're looking at a closeup of these are all limestone. They were deposited underwater. First, the Muav was deposited, but then it was raised above the ocean, and a river caught into the rocks and gave us that erosion surface. Then the sea came up the river and deposited the limestone of the Temple Butte. But then this was eroded on top and came up out of the water. And then it got back underwater and the Red Wall was deposited. Over here, these are radioactive dates. You can't just look at this and know them, but it was 504 million years ago that the Muav was deposited. Then the erosion, the Temple Butte, the erosion, the Red Wall is only 340 million years ago. And something very similar happened on top of the Red Wall, and that gave rise to the surprise canyon that sits in the Temple Butte position farther to the west.
So we come back, and above the Red Wall, is the Supai. The bottom of the Supai, you may find some limestone with neat fossils like this Trilobite tail or this beautiful snail, this gastropod. Higher in the Supai, you find sediments that were deposited on land, like this fossil trackway. Above the Supai is the Hermit. A lot of them are floodplain deposits. In floodplain, you often find fern fossils like these beauties that were found along the Kyabab Trail in these USGS samples. Then we're going to go above the hermit and look at the Coconino where it lies on the hermit. This one you can see along the trail. Down below there are the river flood deposits, something off the edge of a river that we saw. And above are the fossil sand dunes of the Coconino. Right there is where sand fell down a big mud crack. Think of the Nile River depositing muds and then dunes of the Sahara blowing in and dropping down in this huge mud crack.
Up in the fossil sand dunes of the Coconino, you can find lots and lots of different layers that have little tracks of various creatures, as you see here. These are fairly common. The blue there shows millipede tracks, and there had to be time for the millipede to walk along there. Then we come up here and we're going to look right at the top. It's back to limestone. In the limestone, you find a lot of very interesting fossils. Then we go back, and if you were to go up on top of the Kyabab on the north rim and look north, this is the view when you get the north side of the Kyabab plateau. And if you make a diagram of that, the rocks of the Grand Canyon slant down to the right, and there are younger rocks at Zion on top of that, which slant down, and there are younger yet rocks at Bryce. And on top of those younger rocks of Bryce, we have these old trees, the archeological sites, and all of human history. It's a truly wonderful history. The oldest rocks you can find at the canyon are dated by radioactivity at about 1.84 billion years. So the canyon gives us about 40% of the history of the Earth of 4.6 billion years. So the whole thing is certainly not here at the Canyon. But I hope you see how much fun a geologist has reading this history and how rich and wonderful the history is.
The figure below is a static image of what you saw in the "Grand Canyon Strata" video above. Take a look at it and see if you could explain it to a friend.

The marine Redwall Limestone was deposited next, so-named because it makes a red wall. The limestone is actually gray, with the red from rust and clay dripping down from red rocks above. The Redwall Limestone contains fossils of corals, sea lilies (crinoids) and shellfish (brachiopods), but with notable differences from the fossils of those general types found in limestones below, and both sets of fossils differ from those in limestones above. The Redwall Limestone contains caves and sinkholes, which in turn contain sediments associated with the rocks above. Caves generally form on land or possibly very close to land under shallow water, not beneath the open ocean, so the rocks were lifted near or above sea level and eroded after the Redwall was deposited.
Then, the sea flooded in, at least in the region that would become the western part of the Canyon, and deposited the Surprise Canyon limestone in erosional stream channels in the top of the Redwall. These rocks were not even described until the 1980s, and are only reachable by helicopter or arduous climbing. These Surprise Canyon rocks are not indicated in the diagram, above, which is what you would see on the Bright Angel Trail in the central Grand Canyon, but you could reach the Surprise Canyon by following the yellow arrow out of the picture to the left. Erosion cut the top of the Surprise Canyon before the deposition of more layers.
Next are sandstones, siltstones and shales, called the Supai Group and then the Hermit Shale, with plant fossils, lizard and other footprints, etc., at various levels through the rocks, indicating deposition on land in floodplain conditions. Insect fossils appear on the upward trip through the rocks, and then great dunes of the Coconino Formation with sand-blasted, wind-frosted grains and occasional lizard footprints. You might imagine the sand dunes of the Sahara spreading across the flood plain of the Nile River for these rocks. Marine conditions then returned with the Toroweap and Kaibab rocks, providing mostly limestones with sponge fossils and shark’s teeth as well as corals, crinoids and brachiopods, finally reaching the top of the Canyon.
If you're on the North Rim of the Canyon, gaze farther north. The rocks you're standing on slant downward to the north, and you are looking at rows of cliffs with younger rocks, up through the cliffs of Zion from the age of the dinosaurs, up through the lakes of Bryce from early in the age of mammals, up and up and up until finally you reach the trees and Native American sites older than the historical chronologies of Archbishop Ussher.
(By the way, if you’re interested in the carving of the Grand Canyon, have a look in Module 10 Enrichment.)
How Old is the Grand Canyon?
How Old is the Grand Canyon?
A pile of rocks like those in the Grand Canyon does not reveal its age easily. But we have evidence of seas, mountain building, mountain erosion, more seas, more mountain building, more erosion, and more, and more, and more. The rocks involved are old friends—similar things are forming today. Using the principle of uniformitarianism—the present is the key to the past—we can make some estimate as to how long events take. The schists at the bottom were buried miles deep in mountain ranges and later brought to the surface by erosion, and even relatively fast erosion requires a million years to strip off a mile across a large landscape, for example.
The geologists of the 1700s, working primarily in Europe, pieced together stories such as this. They tried to estimate the times involved. One difficulty was that they could not tell how much time was in the erosional time gaps, or unconformities—was erosion fast, or slow? And they could not really unravel all of the stories in the oldest rocks because metamorphism had erased some of the stories.
These early geologists eventually estimated that the rocks told of events that required AT LEAST tens of millions of years to hundreds of millions of years. Just depositing the sedimentary rocks would take about that long, with much more time represented by the unconformities and the oldest really-messed-up rocks. This is deep time—the Earth is not just the historical thousands of years, or even the tens of thousands of years of ice layers and tree rings. History was written and trees grew on the relics of vastly greater histories. Looking back into that history, like looking over the cliff at the edge of the Grand Canyon, is one of the great joys of geologists. We live in a four-dimensional world, height, width, depth and history through deep time. We hope you are learning to enjoy some of this view over the cliff of time. In the next section, we will see just how high that cliff really is.
Radioactive Clocks
The techniques of layer counting and uniformitarianism are useful in dating, but the real workhorse these days is radiometric or radioactive dating. The Earth includes many different naturally occurring radioactive elements. An atom of a radioactive element eventually will spontaneously change to some other type of atom, by emitting radioactive energy, in ways that physicists describe and predict with incredible accuracy using quantum mechanics.
Radioactive decay occurs in various ways. The easiest to understand is when a nucleus splits into two parts, kicking out a part of itself. Remember that heat causes molecules in water to bounce around and occasionally evaporate; atoms or molecules in rocks are also bouncing around, but are so tightly bound that very, very few break free at the Earth’s surface. In a vaguely analogous way, the protons and neutrons in the nucleus of an atom are always wiggling and bouncing around; most nuclei are so tightly bound that this wiggling doesn’t change anything, but some types of nuclei are weakly enough bound that occasionally some protons and neutrons “evaporate.” We call those types of atoms that “evaporate” radioactive, and those that do not stable. (A real nuclear physicist would probably yell at us because we oversimplified too much, especially because radioactive decay properly is a quantum-mechanical process and not really like heat, but we hope this will do for introductory geology. We can guarantee that there are physics professors who would love to teach you about the real physics of this.)
Commonly, a nucleus that “evaporates” emits a group of two protons and two neutrons, which is the nucleus of a helium atom and also is called an alpha particle, for historical reasons. Other types of radioactive changes also occur, including the splitting of a nucleus into nearly equal-sized chunks, the change of a neutron to a proton plus an electron that is emitted, or the capture of an electron by a proton to change into a neutron. All of these change the type of atom from one element to another. All are explainable by well-known physical principles, and all are as natural and regular as the downward fall of your pencil if you drop it off your desk.
The behavior of any one atom is not predictable, but the average behavior of large groups is easily predictable with great accuracy. Suppose you start with a sample containing some atoms of a radioactive type, and you watch for some specified time such as one hour, or one year. The basic rule of radioactive decay is that you will see more radioactive atoms decay if you started with more radioactive atoms. (Really, it is that simple. We give you the math in the Enrichment, in case you want to prove it to yourself.) If you start your stopwatch when you have some number of a given type of radioactive atom, and stop the watch when half have changed, you will have estimated the half-life of the radioactive type. Each radioactive isotope has a distinctive half-life, which can be measured in the laboratory. (Note that you do NOT need to wait for an entire half-life to measure it. As shown mathematically in the Enrichment section, you need to wait only long enough for enough atoms to change to be measured accurately, a useful result when dealing with types that have long half-lives.)
Suppose you start with 2000 atoms of the parent type. These decay into offspring (most textbooks refer to these offspring as daughters). After one half-life, 1000 parent atoms remain and 1000 offspring have been produced. After another half-life, half of those 1000 parent atoms have changed to offspring, leaving 500 parents and giving 1000+500=1500 offspring. After a third half-life, half of the remaining parents have changed, so that now only 250 parents remain and 1500+250=1750 offspring have been produced. During the fourth half-life, half of the remaining parents decay, leaving only 125 parents and giving 1750+125=1875 offspring.
Now, we really need to deal with large numbers, so add ten zeros to the end of each of the numbers in the previous paragraph. Such numbers of radioactive atoms are common in even relatively small samples of rock; the total number of atoms in a fist-sized chunk of rock is about 1 followed by 24 zeros.
As noted, there are many different parent types with different half-lives. Some half-lives are very short—seconds or less. Others are very long—billions of years or more. Some of the radioactive parents are left over from the explosions of stars that produced the stuff of which the Earth is made. Other radioactive parents are created by cosmic rays that strike atoms on Earth. Some radioactive decays produce offspring that are themselves radioactive parents for a further generation, and several such decays may be required to produce a stable offspring. And radioactive decays may damage neighboring atoms, producing new radioactive types.
Telling Time

Consider the example of potassium-40 and argon-40. Argon-40 has 18 protons and 22 neutrons in its nucleus, for a total of 40 particles. Potassium-40 has 19 protons and 21 neutrons, also totaling 40. Potassium-40 is a parent with a half-life of 1.3 billion years. Potassium is abundant on Earth, and occurs in many common minerals, and some of the potassium is the radioactive parent potassium-40. The offspring, argon-40, is a gas. If lava flows out on the surface of the Earth, the argon escapes. Thus, a lava flow will start with some parent potassium-40 but no offspring argon-40. As time passes, the potassium-40 breaks down to argon-40, which builds up in the rock. If today the rock has as many potassium-40 as argon-40 atoms, then one half-life has passed since the lava cooled, and the rock is 1.3 billion years old. Whatever the ratio is, the math is not that difficult and gives the age.
It is possible for argon-40 to leak out of the mineral. If it does, we will think that the lava cooled more recently than it really did. But if leakage is occurring from a mineral grain, then the outside of the grain will contain less argon-40 than the inside does, and this can be measured, revealing the problem. A mineral grain that grew in slowly cooling melted rock far down in the Earth and that then was erupted may have begun trapping argon-40 before the eruption occurred, in which case the age obtained will be the time when the grain started growing rather than the time when the eruption occurred. Scientists do not blindly apply dating techniques; they think about what is being measured, and apply a little common sense.
Keeping Time
Clearly, we can test radioactive dating against written histories and annual layers, and we can test against the sort of uniformitarian calculations that the early geologists made on how long it would have taken to deposit the rocks we see today. Furthermore, we can test different radioactive isotopes against each other—a rock can be dated by potassium-argon, but also by others including uranium-lead and rubidium-strontium. All of these agree beautifully; the ages assigned to geologic events are based on multiple independent techniques that yield almost exactly the same age for those events.
In some of the stranger corners of the internet you may find people suggesting that maybe radioactive decay occurred at some different rate in the past, and even some of the freer-thinking physicists have suggested slight changes in physical “constants” over time, perhaps affecting radioactive dating. We can be confident, however, that no large changes have occurred that would significantly change the results discussed in this course. The agreement among written histories, annual-layer counts, uniformitarian calculations, and multiple independent radioactive techniques does not allow major changes. Furthermore, because radioactive decay depends on the forces controlling the stability of atomic nuclei, and those forces are involved in all sorts of other processes including energy generation in the sun and other stars, any major change in the radioactive decay in the past would mean that we would not be here today—the sun would have turned off or blown up already, something we know did not happen. (See the Enrichment if you want a little more on these topics.)
The Age of the Earth
The oldest rocks found on Earth are about 4 billion years old, and some of those contain mineral grains recycled from slightly older rocks. The active Earth has almost certainly erased the record of its very earliest rocks. Meteorites probably formed from the solar nebula at about the same time as the Earth did, and since then have fallen on the Earth. The oldest meteorites are about 4.6 billion years old, and that is our best estimate for the age of the Earth. Careful analyses of the changing lead isotopic ratios over time (from decay of uranium) also yield that number for age of the Earth. And 4.6 billion years is, indeed, deep time.
Vintage Video: Supergroup Part 1 (2:02)
This video takes you "live" to the Grand Canyon Rim (on a very windy day), where you will join Dr. Alley in a firsthand look at "deep time." (If that clip leaves you wanting more, "part 2" is also available as an optional enrichment). So, enjoy your visit to the Grand Canyon and your walk up through time. We hope you find Dr. Alley's play-by-play commentary and his incisive post-game analysis helpful in explaining what the Earth has been doing these past 4.6 billion years.
Click here for a transcript of the Supergroup Part 1: Grand Canyon Rim video
The really cool thing here is how much extra time we can see. If we look down just to the left of where we see the river in the shadow of the cloud right now, we'll see that there are layers that are slanting. And then above them there are layers that are horizontal. Now the slanting layers are the Grand Canyon Super Group.
They are rocks that were deposited between about 1.2 billion years ago and about 0.7 billion or 700 million years ago. If you add up the thicknesses of all of those going down it's almost three miles of sediment-- almost three miles. Now we've got a mile on top and then from our feet down to the unconformity.
Then there's three miles of sediment under that. And then if we peer down the canyon in that deep cut down there are the the old crystalline rocks, the old beautiful rocks that were cupped in the heart of a mountain range that are lava flows and sediments that add up to many more miles of rocks. And those have been cut. And that it was eroded. And then these were put on top.
And then faults that are sort of like Death Valley faults broke and dropped these down. And then it eroded on top. And then these were came And then those were deposited. And then those were eroded away. And then the river cut through. And it's so cool. And it's just this immense story that just keeps being told over and over and over.
Optional Enrichment
No, this vintage video won't be on the quiz!
Vintage Video: Supergroup Part 2: Grand Canyon Rim (2:44)
OK, we're here at the desert view outlook, looking out over the eastern end of the main part of the Grand Canyon National Park. But there's some spectacular scenery here that's somewhat unappreciated because here you can see some of the stuff in between, and what's cool about it is that because it's been tilted over, in a sense, you've gotten real lucky and you've preserved a lot more of it than you would if it stayed all vertical. And the reason for that is if you can imagine each of these layers is part of the Grand Canyon super group, and this is just a whole bunch of layers of volcanic and sedimentary rocks. And these are real old these are all 700 million to 1.2 billion years old, something like that.
So you've got these really old rocks that are all lying nice and flat, nice and horizontal, laid down like that, but at some point they tilted over at a slight angle, and then this nice line cut across and started to erode away across them. And so at one end of the canyon see a little bit of this. As you drive to the east you start to see younger, and younger layers coming out underneath what's called an uncomformity.
And then all of this was sliced off. But what's amazing about it is the amount of rock that would have been here a long time ago, it's probably three miles worth, right now it's spread out across probably 20 or 30 miles of the canyon, but originally there was a good three miles of this stuff.
And the fact that they are slanted really helps us to preserve all the different layers. If it stayed flat you'd have sliced off so much more and you'd have lost all that information. It's slanted a little bit, like a deck of cards that's been pushed out and so even though you're starting to slice it off you still retain some of the older decks, some of the deeper parts of that deck of cards.
Optional Enrichment: Deep Time
Optional Enrichment: Deep Time
The big picture on climate and energy is a little too big for our course—indeed, Dr. Alley has been the primary author of a different course on this topic, wrote a book on it, made a three-hour PBS miniseries, and has given more than 1000 public talks on the subject. Here, as Enrichment, we’ll give you some of the highlights, emphasizing the ability of people to solve problems, discussing how important energy is to our well-being and the great value we have gotten from fossil fuels, discussing how the CO2 from fossil-fuel burning is changing the climate, exploring some of the threats if continue with our current energy system, presenting the strong reasons why changing sooner rather than later will make us better off, looking at some of the solutions we could adopt, and saying a few words about communicating these issues. The biggest picture is that, if we seriously work to solve these problems, most people who view this material should live long enough to see us build a sustainable energy system, powering everyone essentially forever, and giving us a larger economy with more jobs, improved health and greater national security, in a cleaner and more ethical world. And that’s good news!
A few of the images are not in the public domain but are used here following many public presentations, with attribution for non-profit educational purposes under fair use. Most of the images are in the public domain, and many (including all of the penguins, which are included mostly to lighten the mood) were taken by Richard or Cindy Alley.
Video 1: The Value of Optimism on Climate and Energy (2:50 minutes)
Dr. Richard Alley, College of Earth and Mineral Sciences, Department of Geosciences: "We're going to start with a little on the value of being optimistic on climate and energy. Let's be honest in the big picture on climate and energy, uh the news is not always good. But before you start jumping to bad conclusions, uh consider this uh like many, many other people I helped the United Nations on energy and climate uh with the IPCC, the intergovernmental panel on climate change. This is us in Paris in 2007 (photo of Dr. Richard Alley and Colleagues at in Paris for the IPCC), the year the committee was awarded the Nobel Peace Prize. If you took the tens of thousands of pages of IPCC reports, and you squeeze them into 35 words."
Dr. Richard Alley: "Compared to business as usual, efficient responses on climate and energy will give a larger economy with more jobs, improved health, and greater national security in a cleaner environment, more consistent with the golden rule. This really is correct; this is what the scholarship says. The good news is if you're a young person today, you're part of the first generation in human history that can build a sustainable economic energy system that'll power everyone essentially forever. But we have to remember that we can solve problems and then go out and solve them. And we can solve problems this is a cell phone (holds up cell phone), and I have a picture here of a do-it-yourself cell phone kit. It's just a little bit of quartz or sand for the glass (circles photo of sand on screen), and it's a little bit of organic material such as oil for the plastic (circles photo of oil on screen), and it's the right rocks, the ones with the rarer elements and the Palladium and such (circles photo of rocks on screen). And that's all it is, is sand, oil, and rocks. And science, and engineering, and design, and marketing, and banking. There's a GPS in here that knows where you are. It has relativity special and general relativity from Einstein. If it didn't have those it would begin to get lost in 2 minutes. It has quantum mechanics in the computer. If we can do this, we can surely do energy. So, in this set of short videos, we'll discuss the big picture, the nature of the problems, and some of the possible solutions. Let's go see."
Video 2: The Value of Energy (13:58 minutes)
Dr. Richard Alley, College of Earth and Mineral Sciences, Department of Geosciences: "Here's a little bit about the value of energy. Dealing with climate and energy is hard because energy use is so valuable to us, and right now most of our energy is from fossil fuels. Here's a little bit of history and what's going on."
Dr. Richard Alley: "A human diet:, what we get from our food that allows us to do things: To run, and jump, and hoe, and what have you. We eat about 2,000 calories per day. If you burned your food over 24 hours, the energy coming off is just 100 Watts. It's one, old light bulb. A Tour de France rider can do a few hundred Watts, but they're eating 10,000 calories a day. What we can do is not that much. What is done for us though? We don't have to light our light bulbs. We don't have to cool and heat our rooms by generating the energy from our food. We have air conditioners, and we have tractors, and trucks, and all this wonderful stuff that's done for us. In the United States, what is done for us from outside is 100 times more than what we could do for ourselves. Averaged over the world, it's about 25 times. We really love this, our well-being depends on it. And it is still more than 80% fossil fuels in the US, and in the world. That's why this is hard."
Dr. Richard Alley: "You can tell the history of humanity from so many different ways. And from the common workers, and from the leaders, from our art, from our religion. You could tell the history from our use of energy. Ever since the discovery of the control of fire, we have this long history of energy crisis. We find something to burn to get energy from to do our work for us, we burn through it much faster than nature makes more, we suffer very large unintended consequences, we get sick and other sorts of things, then it becomes scarce. We have intrusive governments, we may fight wars over it, and then we find something new to burn, and we do it over again. And here's the history from Penn State's view. If you drive into State College from the East, headed towards Penn State University, you go past the reason that Penn State is there. Because Penn State was founded by the iron masters, up the hill, from the iron furnace. And the furnace was put here because there was Red Dirt that you could get iron out of, there was limestone flux, there were trees for charcoal, and there was a stream, a spring, a water source that could drive the water wheel that supplied the blast to make the furnace hotter."
Dr. Richard Alley: "This tremendous picture (Figure 11: Civil Engineering Students Taking Velocity Measurement on Thompson Run) is early on when Penn State had been founded, which has students in civil engineering in their ties and their hats, gauging the outflow of the spring, that is why the furnace was put right where it is, to learn how to do this important task before they became engineers."
Dr. Richard Alley: "We go back to the furnace (picture of iron furnace in State College). When the furnace was operating, it would have looked like this (image of Hopewell Furnace in Philidelphia, Pennsylvania). This one is Hopewell Furnace down towards Philadelphia, and it is turning that red dirt into the iron that was used to build the East. The water wheel is over here that would have been driven by our spring, and it's burning. And to do the burning you've got to be dumping in things to burn, and what was it burning? Charcoal. Near here we have something called Collier lake. Colliers or the Colliers (different pronunciation), were the people that turned trees into charcoal because charcoal was just able to burn hot enough to smelt the iron, whereas the trees were not. They make this giant pile of logs, then they'd cover it with dirt. This is actually a demonstration (picture from the US National Archives, reenactment of people making charcoal), these people did this job when they were young, and they were now showing in their old age, they were showing a photographer how they used to do it. You bury the trees in dirt, you burn them with reduced oxygen, drive off the water and some other things, hope that the dirt doesn't break so someone has to climb up there and fix it and try not to fall in and die, and eventually it makes the charcoal that allows you to smelt the iron. And it did this (photo of individuals reenacting logging for charcoal, showing a field of chopped down trees). An iron furnace, and the people who took care of it, think about a square mile of trees per year to make it go. If you had an open forest and you could walk for 20 minutes. and then turn and walk 20 minutes. and then come back. that would be enough trees for one year. And then it's maybe 50 years until enough trees would grow back that you could do it again. You need this huge quantity of trees."
Dr. Richard Alley: "Now if you go to a map of Pennsylvania today, and ask where is furnace still on the map? Every line on your left here (list/graph of Pennsylvania furnaces by hometown), is a furnace that we still remember and there are many more furnaces that are back in the woods that we've forgotten on this map. And every one of those, when it's running, is a square mile of trees per year. They made something called pig iron that then was shipped to four forges, like Valley Forge, where you made valuable things from the pig iron. Every one of these on the right (circling list of forges with furnaces on Pennsylvania list/graph), is a forge another square mile of trees. And trees are being used by people who are not making iron, to make their houses, and to heat, and to cook, and so on. And what happened, this ("Penn's Woods" to "Pennsylvania Desert" Picture; indicating trees were chooped down leaving woods bare). Pennsylvania means "Penn's Woods." They said when the first European settlers arrived in Pennsylvania, a squirrel could have gone up a tree on the Atlantic Coast and stayed in trees all the way across to the Mississippi. The first Forester of the Commonwealth of Pennsylvania was someone named Rothrock, and around 1900 he wrote about the great Pennsylvania desert. Now it wasn't a desert, we still had rain, but we didn't have trees. We had cut them all down. We now have a million deer in Pennsylvania, there might have been a few left. We reimported elk because we got rid of them, we got rid of the bears, we got rid of the nittany lions. There wasn't a deer to eat, and there wasn't a tree to do it behind. We just use groundhogs for Groundhog Day. We couldn't use bears or something else large because we got rid of them."
Dr. Richard Alley: "It was not just us. If you know Cape Cod, you know so Cape Cod sort of sticks up there off the coast, and right about here on Cape Cod (Dr. Richard Alley indicates coast with his arms/hands) is where the pilgrims first met the native people in Eastham. They said it was so goodly a land and wooded to the brink of the sea. Still in the 1600s the town, of Eastham outlawed the ability of people to cut their own trees on their own property. There weren't any left. Deforestation was so extreme that they panicked, they didn't know what to do. It was the row walks the cape in the 1800s and he wrote: "Many of the people get all of their fuel from the beach. If there is a shipwreck, you can burn it. If a tree drifts over from Maine you can burn that, otherwise you can't cook dinner." Over in Manhattan still in the 1600s, the common council is passing many laws on rights to wood, fair trade in wood, they're paying inspectors to make sure you're getting what you paid for. If you don't like government, you better not get into scarcity, because we demand governments to help us at that time when we have shortages and we hit energy shortages very early."
Dr. Richard Alley: "Now, if you've ever tried to read by fire light in a dark, Pennsylvania winter before electric lights are invented, it's not very easy. You're not going to do 12 stitches to the inch on your quilt in that flickering light of a candle or a fire. So, what did you do? Poor people burned a biofuel a mixture of alcohol and turpentine, it was fairly cheap, it was good light, but it was explosive. And there's horrible stories you know the Methodist Minister and his wife go out to visit the parishioners, and the daughters try to refill the lamp, and it blows up and burns them to death. And so rich people, burn whales. The whale oil was clean, and it was bright, and you didn't have it exploding. So, you take large quantities of money, you ship it to New England, and they put sailors on ships, and they go out and kill whales. This is the history of whale oil production from the Yankee Fleet from 1800 to 1880. And at the peak, there are 10,000 sailors out of New England trying to kill whales. This is a complicated story. The fleet got crushed in the sea ice off of Alaska, the insurance went through the roof, and that could shut down some whaling, but what were they doing in the sea ice off of Alaska? They couldn't find any whales that they could kill closer. They had basically killed all the whales they could find. There were some whales that were too fast for these sailing ships, and various other people from Japan, and from Norway, and Russia, and what have you killed them with diesel and harpoon cannons. When we finally quit whaling, there was no economic resource of whales left. We had killed so many whales that there just wasn't much left in the ocean. As they got good at whaling, they drove the price down. That low spot there (pointing at dot on graph indicating $7/gallon of whale oil) was about $7 a gallon in modern equivalent. When they hit the peak of whale oil, not when it was totally gone, but when they hit the peak, it's up to about $25 a gallon. Just this huge, huge price increase, not when it's gone but when it was half gone. If you took a hundred years of Yankee whaling, all of that oil, 10,000 sailors at the peak, you put it into modern tankers, and you replace the petroleum we're using, it would last the United States 11 hours. The idea that we go back when we run out of oil and do the things we used to do, is laughably absurd. We cannot do that; we need something better."
Dr. Richard Alley: "But we do have trees, and we have whales, because we switched to burning fossil trees and fossil algae. This is not a new idea. This is an editorial cartoon (showing photo of "Grand Ball Given by the Whales in Honor of the Discovery of the Oil Wells in Pennsylvania"), it was published in the magazine Vanity Fair in the year 1861, just before the US Civil War. And you can see the title, it was the Grand Ball Given by the Whales in Honor of the Discovery of the Oil Wells in Pennsylvania. So just before the US Civil War, the oil wells of our native land, may they never secede. Oils well that ends well, we whale no more for our blubber! We saved whales with mineral oil, with petroleum. This is a piece of sheet music (photo of the American Petroleum Polka) from the year 1864. Tt's the American Petroleum Polka. We're going to dance to oil wells. It says at one point, this oil well through pure oil 100 feet high, so there comes oil. Oil was always black. It is black. It will always be black. But with her wearing her white top here (woman in photo dancing with white top), they didn't want black oil falling on it so they made it white."
Dr. Richard Alley: "When we quit burning so many of them, a lot of the trees, and a lot of the whales, grew back in a hundred years. When we quit burning fossil fuels, nature will make more in maybe a 100 million years. The rated formation is so close to zero that you can just set it to zero. We must change. We cannot decide between say renewables and fossil fuels. Either we burn and then we learn, or we learn while we burn. And we are confident, if we burn before we learn, we will change climate in ways that we really don't like."
Video 3: The Problem of Human-Caused Climate Change (12:01 minutes)
Dr. Richard Alley, College of Earth and Mineral Sciences, Department of Geosciences: "It's worth taking a little time to discuss why human-caused climate change is a problem. It is true that politicians and the public are still debating whether we humans are causing global warming, whether global warming is happening, whether it's really fossil fuel burning that's doing it. But the fossil fuel burning releasing CO2, changing the atmosphere, changing the climate, affecting us, is really undeniable fact now."
Dr. Richard Alley: "So let's take a little bit of history. We'll start over here in 1800, and run through today with a timeline, and look at a few events, some of which are connected to my cell phone and its development. So, in the year 1900, Planck introduces quantum mechanics. And you know that the computer in my cell phone is designed using the principles that come out of our understanding of quantum mechanics. In 1905, Einstein introduces the first part of relativity, special relativity. My cell phone has a GPS in it, and the GPS is using both special and general relativity because the satellites are higher in Earth's gravity well going faster than we are, and it works. You know this is a wonderful thing. In 1915, Wegener introduces his ideas on continental drift, and we geologists use our understanding of continental drift as one of many ways to help us find or deposits that go into getting the things that you use to build the cell phone. Now the cell phone has a nice person in there, there's a lady's voice that tells me where I am, and she is using filters to hear the signal from the satellite. And those filters are based on mathematical work that was done by the great French mathematician Fourier. In the year 1824, Fourier started calculating the balance of energy for the Earth, and he said the simplest model leaves us colder than we really are. But the simplest model has to be about right, so something else must be going on. And he suggested that there's something happening in the air that warms us in a way that's similar to how a glass plate on top of a sunlit box will keep that box warmer. And we now call this the greenhouse effect. So, Fourier introduces the greenhouse effect in 1824. The role of CO2 was first identified by Eunice Foote in the US, and then there's a lot more work done on this by Tyndall in the UK 1856-1859. The first calculation that's accurate of the warming of the surface that will be caused by humans burning fossil fuels, is by Arrhenius in 1896. So, climate is not some newfangled, speculative thing that's going into building the cell phone that's in your pocket, but it is really long-established science."
Dr. Richard Alley: "Arrhenius's calculation was done with classical mechanics, it was before Quantum. Our Quantum understanding is in many ways based on work that was done by the US Air Force after World War II. They wanted to do communications, and operations, and heat seeking missiles. If you're going to target the hot exhaust of an enemy bomber by looking at the infrared radiation coming from it, if you use the wrong wavelength on your sensor, CO2 absorbs the radiation, and your missile will not hit the target. And I can tell you today, wherever you are in the course of the day, there will be more infrared radiation going up from the sun-warmed Earth, than coming down from enemy bombers. And some of that radiation is absorbed in the atmosphere. Before we ever had satellites, the physicists predicted what those satellites would see when they looked down, and how it would change over time, and those predictions have been successful. So that blue line (pointing to blue line on graph showing the increase in wave number and temperature energy to space to cool Earth) is following the predicted and observed radiation going up from the sun-warmed Earth to be seen by satellite. In fact, that has changed over time as we've changed the atmosphere and the temperature of the Earth, so it's up just a little bit here's two lines on there. But maybe the most important thing here, is that that huge divot is energy that is kept in the earth system because CO2 absorbed it. And if we have more CO2, more can be absorbed. You meet the person who says oh I don't believe in this. They don't believe in the ability of the Air Force to build a heat seeking missile? This is physics, we raise CO2 in the atmosphere that has a warming influence. It's just physics. It is successfully predictive, and I'll show you just a little bit of a vast amount of data. Here's a plot starting in 1880 and coming up towards the present of different temperature records developed for the surface of the Earth, by different groups with different funding (showing temperature anomaly graph from different 5 different agencies). Including one of these groups that started from a physicist who sort of didn't believe that the other scientists had done their job right, got money including private money to demonstrate that the temperature records weren't good, and the temperature records were beautiful. This one show, (pointing to graph with arrows) that sometimes even a little more warming than the others but you can see all of the data agree with the temperature wandering up rapidly, because we're driving it up."
Dr. Richard Alley: "Now, that's thermometers. If you simply restrict yourself to thermometers that are far from cities, they also show the warming. Thermometers placed in the ground, show that the ground is warming. Thermometers placed in ocean water, show that the ocean is warming. Thermometers taken up on balloons, show that there's warming above the surface. Thermometers looking down on satellites, show the warming. If you look at temperature sensitive snow and ice, not South Pole. I've been to South Pole it's minus 50 if it warms up to minus 40, it won't melt yet. But if you look at the temperature sensitive ice: springtime snow, lake ice, river ice, sea ice, temporary frozen ground, um perm frost (permanently frozen ground which is now not frozen), and places the mountain glaciers, the edges of ice sheets, all of these are being lost with warming. If you ask plants and animals where they're living and when they do things, the vast majority of the shifts are in the direction you expect from warming. So as early as the 2007 UN report, warming in the climate system is unequivocal."
Dr. Richard Alley: "Now how do we know that the warming that must happen from the CO2 is what is driving the warming here? Well we've looked very hard for other causes, and no the sun has not brightened over the time we're looking at it wiggles a little. Nothing else is going on to drive this, and the CO2 predicts it beautifully. So this plot which comes from the director of the NASA Goddard Institute for Space Studies (showing graph of forecast evaluations for models run in 2004), has on it both data and model output, and it's it starts over here on the left where the models have been told about things like the particles from volcanoes blocking the Sun, and making that little cold divot there (showing dip in graph between 1990-2000), that was Mount Pinatubo. And then it runs into the future, we don't know when the volcanoes will erupt, so there's nothing put on there but there haven't been any really big volcanoes in this interval. So that there's not a huge volcanic influence. What the models say is that our CO2, the other changes we're making but mostly CO2, will drive warming. Every model run has in it some variability from things like El Ninos, but more than a few years in the future you can't predict El Ninos exactly, it's like predicting the weather exactly. And so what's happened in this plot (indicating Forecast graph on screen), is the models have been averaged to give the black line (on graph), and what you should expect is the black line plus a little bit of wiggling from El Ninos. What then happened after this was predicted, is just what was predicted. This is successfully predictive for the warming, for the pattern of the warming. The stratosphere cooling as CO2 holds heat down here, more warming at the Arctic, more warming on and then the ocean, and other sorts of things. The pattern the amount of warming is indeed being successfully predicted and we know this."
Dr. Richard Alley: "Now this is a particularly interesting one. It starts um further back in time, it goes further into the future than we've gone. On here our projections and predictions and actual data, and this happens to be scientists who we're working with, or for funded by, oil companies. So, each of the gray lines (on graph) starts at some time well in the past. That one was way back, others are a little closer to today, and each one of them is one of these. This is what we'll get if we keep burning fossil fuels into the future for an oil company, and then the red (line on graph) is what happened. This works. It really, really, really does work. The science is solid, we are driving this."
Dr. Richard Alley: "The warming effect of our CO2 is not the least bit surprising, but what is surprising Arrhenius said I don't think we'll burn that much fossil fuel. That was what's really surprising is how good fossil fuel companies have been at getting us what we burn. There's a comparison here. You know what trash looks like, you've taken out the trash for your parents maybe, or done it for yourself, or try to get kids to do it for you. In the US, we throw away at the curb for the trash collector a half a ton of trash per person, per year. And the US averaged over sort of the early part of the 21st century was about 16 tons of CO2 per person, per year. That's dropped a little maybe even a shade below 15 now, but it's still 15 tons per person per year and it is changing the climate."
Video 4: The Dangers of Not Changing our Energy Systems (6:39 minutes)
Transcript coming soon!
Video 5: The Benefits of Changing our Energy System (6:39 minutes)
Dr. Richard Alley, College of Earth and Mineral Sciences, Department of Geosciences: "Now, we can have a look at some of the benefits of building a sustainable energy system because there really are large benefits available if we take actions now, to avoid the worst damages from a warming climate."
Dr. Richard Alley: "If we do this well, compared to business as usual, we get a larger economy. More jobs, we're healthier, our nations are more secure, the environment is cleaner and were more ethical. I'll show you one slide on each of these. This comes from the Nobel prize in economics in 2018, William Nordhaus, I actually had the honor of serving on a committee with him, an amazing person. He was awarded for building tools that provide guidance to policymakers and the general public on how to make the economy help more for more people. We have resources, we can use those resources for consumption now to help people feed them, and clothe them, and house them, and so on. We can invest broadly in the economy to make it bigger in the future to help more people. We can target those investments on particular issues, such as climate change. When these models are run, they keep saying over, and over, and over, that we're not in investing enough in slowing down climate change now to make the economy be its best. You may meet the person who says oh yeah, the climate is changing, but we can't afford to deal with it. The Nobel prize in economics says you can't afford not to deal with it."
Dr. Richard Alley: "Now a bigger economy is likely to give more jobs, and that's especially true if we shift away from fossil fuels Now, I worked for an oil company one summer, great people, many of our students have gone to work for oil companies, but it's fairly clear that any other good approach to making our energy system makes more jobs. And the reason is at least in part, that the cheapest oil in the world, which maybe from some other country, can probably be produced for about $5 a barrel. And that goes to jobs, but we may be paying $75 a barrel or some other large number, because oil is scarce. And the money is going to those people or countries that control the scarce resource, not to the workers who make that scarce resource useful. The Sun, the wind are just about everywhere, so if we switch to those, more of the money of the energy system goes to good jobs, not to controlling a scarce resource.Now if we make it too hot to live in places, that is very unhealthy. There was a recent study that found that 20% of deaths globally are linked to breathing particles that come off of burning of fossil fuels. Making more forest fires is not going to be good flooding people's houses, and then spreading diseases is not good. So, all of these medical groups in the US got together and they said, if we want to be healthy, we need to go on the inevitable transition to clean renewable energy."
Dr. Richard Alley: "Here's a picture of rear Admiral David Titley from our earthy operators manual PBS miniseries. Admiral Titley is a Penn State grad, he came back and taught at Penn State for a while, and truly amazing, brilliant wonderful person. And after his retirement he served on the CNA military Advisory board, with a large number of other military leaders. And they said, you know if we make it too hot to live in some parts of the world, this will drive climate refugees across borders. It's not good for them. It's not good for the security of the countries. If we flood our navy bases with sea level rise, that's not good for the security of our country. If we want secure nations, we need actionable agreements on ways to stabilize the climate. That's our military leaders."
Dr. Richard Alley: "We're increasingly driving climate change that forces species to move. Some of them are going up mountains and trying to track after colder conditions, and then they get shoved off the top of the mountain and they're gone. Some of them may be isolated in national parks and they need to go to a different park, but we're in the way. If we don't take actions to stabilize climate, the studies are that roughly a third of the species on Earth could be placed on the road to extinction by the time a typical college student is in the prime of their career."
Dr. Richard Alley: "And then there's the ethical thing. The top picture here (Geographic disparities and moral hazards comparing maps, pointing at CO2 map of the world), the countries in red (North America, Asia, Europe, etc.) are emitting a lot of CO2 per person, per year. The ones in blue (Africa, South America, etc.) not much down here who suffers from the warming. All future generations and poor people in hot places now. And if you notice these two plots (both maps) sort of look like they're backwards from each other. The people causing the most warming are suffering the least from it. I quoted Pope Francis here, that we can't leave this to a future generation. I could quote many other religious and ethical leaders; this does not look like the golden rule. So, if we get a handle on this, larger economy, more jobs, improved health, greater national security, cleaner environment, more consistent with the golden rule. How do we do that? Let's go take a look at a few of the many options."
Video 6: Some of the Possible Solutions (8:53 minutes)
Dr. Richard Alley, College of Earth and Mineral Sciences, Department of Geosciences: "So now, some of the possible solutions for building a sustainable energy system. The solution space is huge. And we're only going to highlight a very few things here, but I'm picking out some of those that are especially likely to help."
Dr. Richard Alley: "Start here (map of the world comparing croplands and rangelands). Everything in color on these maps, we use to get the 2,000 calories per person per day that are the human diet. On top is crop lands, on the bottom is grazing lands where animals eat grass, and we eat the animals, or we milk them. Suppose we decided to replace every bit of external energy used by every human from oil, and coal and gas, and nuclear, and everything else with a modern solar farm. And then we added a little extra to allow growth. That pink square in the Sahara (indicating on map), would be enough to replace all external human energy use. Okay now we're not going to put it all there, some of it's on the roof over my head right now, but that gives you the scale. It's a huge area. It's a huge task. But it's surely doable, and that's the really important thing, and it's small compared to what we've already done to the land."
Dr. Richard Alley: "When we made the PBS miniseries, Earth The Operators, manual we got to go talk to some Texas ranchers, they were having real economic trouble. There was danger of the town going broke, the high school being closed, people losing the ranch. They put in a wind farm, and they get what they call mailbox money. You go out to the mailbox and it's full of money. 5% of the land in and renewable energy, often pays more than 95% of the land in the old ranching. If we put a wind farm on the windy parts of the plains and deserts of the world, the places where the wind blows enough to run the turbans at least 20% of the time, that would be five times more energy than now used by all humans. So, the resource is there. The International Energy agency, they spent a lot of years sort of not being enthusiastic about renewable energy, but by 2020 they got to the point of saying hey it's the cheapest electricity in human history. Cheaper than anything else in many places. Okay? So, the cheapest electricity in human history with a resource that's far greater than what we can use at this point. But there are ways to combine this wisely. This particular experiment was done at Oregon State, it dries out there in the summer, and when they put solar cells in it held some snow underneath early in the year, it kept it a little moisture farther into the summer. They got extra hay cutting because they were also getting extra electricity from the solar cells."
Dr. Richard Alley: "This was an experiment done in Germany. They made High solar cells you could drive tractors under. They planted different crops extending outside and under, so they could compare how it worked. And this summer had a nasty drought, and in this summer, they got more food where they were also getting electricity. So, you're getting more money from the electricity, but they also got more food because of reduced evaporation in the drought. So there are ways to combine these things. Right? There are just times when it's nice to have a little shade, and because the area needed to do this is so small compared to the area we use for agriculture, you could design things that give us the best of both worlds. This particular study said, you know global energy demand would be offset by solar production if less than 1% of crop land is converted to this dual use. We grow food and we eat it. We grow food and we feed it to animals, and we eat them, or we milk them, or take their eggs. We grow food and we burn it. Some of it is ethanol in in gas tanks and we burn palm oil, and other things. Biodiesel. We use more than 1% of the crop land now for food that goes into biofuels, than we burn. We could get all of our energy from that much area. So, somebody says oh we don't have the land to do this, they're not really serious."
Dr. Richard Alley: "You go places that we irrigate. Water is evaporating from the irrigation canals, and that's food that we're not growing. If you put solar over the top of the irrigation canals, less evaporation, more water, more food, and you're getting energy from those solar cells. It's a win-win-win. This is for the US grid. This comes from Lazard, the world's largest independent Investment Bank (graph of cost of energy comparison on screen as of November 2019). They've said suppose there were no subsidies for wind and Sun, how much does electricity cost if you want to add a little to the US grid compared to other sources? And here's a blow up of this (showing from cheapest to most expensive: Utility scale solar, wind, gas peaking nuclear, coal, and gas). Cheap as is over on your left expensive is over on your right, going that way. And what you'll notice here, (circling Utility scale solar and wind) is that the renewables are the cheapest now. Gas generally costs more, coal costs a lot more, nuclear is having real troubles being competitive. Gas peaking is just you run it when you really need it. These two diamonds down here (circling gas peaking nuclear and coal) are if you have an old nuclear plant, or a coal plant, it's completely paid for. It is wired in. All the construction is done, and you're not paying it off. And that's just to operate it. Compared to building and operating new renewables this is why you're seeing coal and nuclear in looking for subsidies from state governments and elsewhere, because they're having real trouble competing with the renewables at this point. Now this would look much better for the renewables if fossil fuels were not subsidized. Because we now have authoritative statements that the fossil fuels are much more subsidized than the renewables are."
Dr. Richard Alley: "So, this is something from people working with the international monetary fund, the IMF has been updating this, and they found that now the subsidy for fossil fuels in the world is more than $7 trillion dollars a year, or more than 7% of the world economy. More or less, when we pay a dollar for fossil fuels, society pays another dollar. Some of this is direct subsidy, tax breaks and other things, mostly it's because when we burn fossil fuel, the cost of the health impacts, the cost of the change in climate, is paid by society not by the consumers of the fossil fuel. And the IMF folks found that if we eliminated this subsidy, we would cut air pollution debts, we would reduce CO2 emissions more than half, we will increase the well-being of people."
Dr. Richard Alley: "Now do not kid anyone. Building a sustainable future is going to be hard, it is going to be decades. We don't really have all of the answers. But we now know that it can be done. And if you're a young person, you're part of the first generation in history that knows that you can build a sustainable energy system, and that doing so can help the economy, and health, and security, employment, environment, ethics. I took this picture in Greenland. Can we really have a world with icebergs and rainbows? Yes, we can. And I think that is really good news."
Video 7: A Few Thoughts on Communications (7:48 minutes)
Dr. Richard Alley, College of Earth and Mineral Sciences, Department of Geosciences: "So, a few thoughts on communications. If the new energy world is so wonderful, why aren't we moving forward on it faster? It's a really valid question."
Dr. Richard Alley: "This came out in 2024 (NATO Climate Change and Security Impact Assessment), from the North Atlantic Treaty Organization, NATO, you're welcome to stop this and read the text if you'd like, but the key part is at the bottom. They found that Kremlin-backed, that is Russian actors, are pushing climate change denialism. They are actively attempting to derail climate change, mitigation policies, and renewable energy Investments. Why would they do that? Well, the world bank has been tracking these things, and they said that roughly $1.5 trillion dollars per year across the world, goes to oil producers. Not for finding, drilling, pumping, and shipping the oil, but because they control a scarce resource. The extra above what's needed to produce the oil is a trillion and a half dollars a year. The cheapest oil producers can probably make it for $5 a barrel, and they're getting something vaguely like $75 a barrel, and that might be a really strong reason why some actors, Russia, and others, could be working to block progress on this. In addition to this grossness misinformation though, they really are issues, that really do matter in the energy transition. The change won't be easy there are things we have to learn yet. There are some things that won't work beautifully initially. It took us a century to learn how to handle most of the explosions, and the leaks, and the carbon monoxide, and all these other problems that come with fossil fuels. Which are really huge and dangerous. It's likely to take us at least 30 years to replace that with something even better. And for example, even though we will get more good jobs by doing the transition right, some people will lose jobs as we switch. And either we take political actions to help them, or we will have some unhappy people, mostly concentrated in certain places."
Dr. Richard Alley: "This map (Overall employment carbon footprints, by county in the United States) was published in the proceedings in the National Academy of Sciences, and it's showing how much CO2 is produced per year by a typical job in each county in the US. So, this is tons of CO2 per year from the average job in each county. The Bluer ones, as low as 1.5 tons. The redder ones, as much as 2,000 tons. Now there is a cost to society of this, the cost to health, the cost of climate change. The paper took an estimate of what this cost is, and then applied it to the CO2 from the jobs. The lowest ones below $400 for a job for the cost of society of the warming from other things from the CO2. The highest ones almost half a million dollars. Alright? So, I hope it's evident, that relatively few jobs produce most of the CO2, but those jobs are very important in some counties. And either we do things politically to help people in the transition, or there will be very strong unhappiness in some places."
Dr. Richard Alley: "Now a few thoughts on communicating this. Please note that the color scale will be different in the next plots, they were not done by the same people. Bluer now is going to be sort of rejecting climate change, and redder is accepting. This is work done by Yale Climate Communications, they are very well respected. A note here, a map is a valid way to look at the US, but it is not the only way. So, if you go up to the dot (showing population map in the United States) that's Philadelphia up there, there are almost three times as many people in Philadelphia as are in the entire state of Wyoming. The Philly metro area, which is more or less that little blue circle up there in the right, has more people than all six states that are outlined in pink there (Alaska, Montana, Wyoming, Idaho, South Dakota, and North Dakota). More people than all six put together. But those states have 12 Senators, Philly does not. Okay, so most Americans accept climate science, you'll see that down below which is the people part (on map). But we'll see that people in most of America, are not as sure about climate science. And so, a map based on area, and not people, can give you a very odd view of the country."
Dr. Richard Alley: "So, this one, to ask, is global warming happening (Another map of the US, circling a county in Utah). There's a couple of counties that said no, but most places said yes, and most people certainly said yes. Is it caused mostly by humans? Most people still say yes, but now huge chunks of the country are saying no."
Dr. Richard Alley: "Do scientists agree? You learned about this from scientists, yet a lot of places think that scientists don't agree, most people still say they do. Somebody probably paid a lot of money, and a lot of effort to confuse the public about this one. Are you worried about it? The worries are actually somewhat higher, that probably reflects reality. Should we look for solutions, should we help find solutions? The moment we move from problem space to solution space, it's a very different view. When you get a job, the boss does not say bring me your problem. The boss says bring me your solution. And that's what America is saying, bring your solution. But are you talking about it with your neighbors? There are one or two counties that more than half of the people are, but that's just about it. We're not talking about it anywhere. This is, you might say this is the Uncle Ed effect. You sit down to Thanksgiving dinner, you say how about that global warming, Uncle Ed says oh it's an evil plot to take away my pickup truck, you say it's okay Uncle Ed, just calm down and pass the potatoes, and how about the Steelers. Right?"
Dr. Richard Alley: "So many people believe that we can successfully solve problems that they deny exist, and you just saw it there. Very few are talking about it. Personally, I think there's a real lesson here, and that communicating on solutions finding the good, as well as the bad, is really important. Because if we do that, we can build a sustainable energy system that will power everyone essentially forever, with a larger economy, more jobs, improved health, greater national security, in a cleaner environment, more consistent with the golden rule. And I think that's great news."
Optional Enrichment: Carving the Canyon and More
Carving the Canyon, and More on Radioactive Dating, and Radiocarbon ages of "Old" Living Clams
Many people are interested in the carving of the Canyon, and the age of the Earth, and related topics. Often, this interest is linked to certain objections to the science of an old Earth, possibly arising from the deeply mistaken idea that a person cannot be a good member of some religions while accepting the science of geology. (Full disclosure: Dr. Alley is a long-time member of a reconciling Methodist church.)
The short essays below address a few of the questions that Dr. Alley has heard in these areas, and may serve as starting points if you have additional questions.
Carving the Canyon
Really big, deep canyons are often found closer to mountain ranges than the Grand Canyon is—it’s fairly easy to cut deeply into something really high, while the river doing the cutting is still high and steep. So why is that immense canyon out there in Arizona, and how long did it take to cut?
A vigorous river is capable of cutting downward at 1 mm/year (or more, and glaciers may cut faster than that). At 1 mm/year, it takes 25 years to cut an inch, or only about 1.6 million years to cut a mile down and make the Grand Canyon. Usually, rivers don’t cut as fast as 1 mm/year because the rivers quickly get down close to sea level, which makes the river’s slope smaller and slows the erosion. But, the Grand Canyon probably took longer than that, as we’ll see soon, in part because the river had to cut several times deeper than the Canyon is!
The Grand Canyon likely owes its existence to several events, including opening of the Gulf of California causing “river piracy”, stealing a different river to run through the Canyon. As we saw way back in Module 2, sea-floor spreading began in the Gulf of California about 5 million years ago, and this likely triggered changes that propagated inland and eventually diverted the Colorado River through the growing Grand Canyon into the Gulf of California. The opening of the Gulf of California brought the ocean closer to the mountains, which steepened the streams flowing into the Gulf—the height of the mountains wasn't changed by opening the Gulf, but the horizontal distance a river had to flow from the mountains to sea level got shorter as the land ripped open.
In turn, this likely led to one of the rivers cutting into a high plateau and eventually cutting through a continental divide and diverting the ancestral Colorado River in an act of river piracy. A “continental divide” is the line on a map separating the rivers flowing to one ocean from the rivers flowing to another ocean, or somewhere else. As you might imagine (and as we discussed briefly back in the history of the closing of the proto-Atlantic and opening of the Atlantic in Module 4), the slope to one ocean from a continental divide is often steeper than the slope to the other ocean. The steeper side generally erodes faster, which causes the continental divide to move away from the steeper side toward the more-gradual side. (Eventually, this will lead to the slopes being similar on the two sides.)
But, the continental divide is irregular, not a straight line. Where a big river forms and cuts down, the slope from the divide to the river will be steeper than nearby, so erosion will be faster there and the divide will be forced away. Sometimes, this will cause the divide to intersect and “capture” the drainage of a stream that had been on the other side of the divide. (See the figure below.)
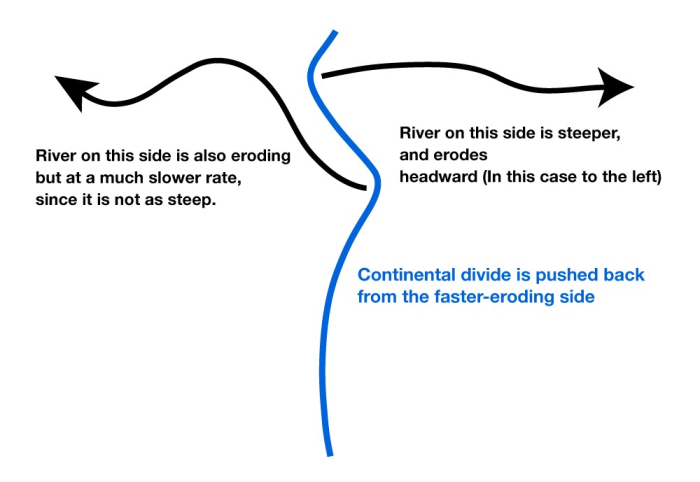
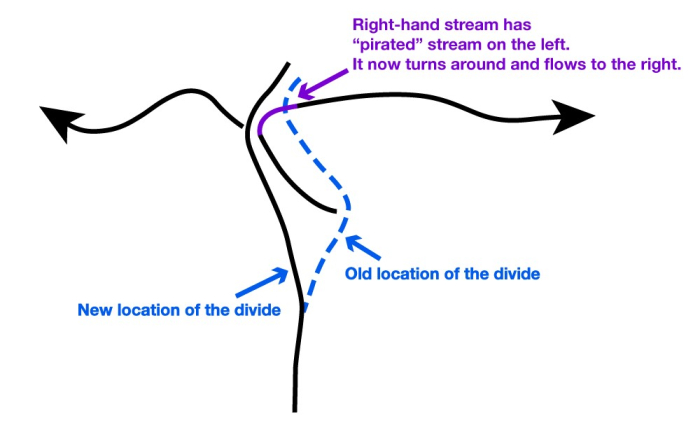
The upper panel in the figure shows two rivers (the black lines with arrows), separated by a continental divide (the blue line), viewed from above. If the right-hand river is steeper, then it will erode back until the headwaters of the left-hand river are “captured” in an act of “stream piracy,” as shown by the purple line.
There are lots of small rivers in the West with fairly big canyons—look at the most-of-a-mile deep canyon of the small Virgin River in Zion, or the remarkable amphitheater that tiny Bryce Creek has gnawed into the Paunsagunt Plateau to make Bryce Canyon. So, when the Gulf of California opened, the ancestral lower Colorado River steepened and cut headward (probably involving a piracy event with a stream exploiting the easily eroded San Andreas Fault), and pirated the ancestral upper Colorado River, which previously probably had drained internally (the river ran out into the desert and evaporated, the way some rivers do in Death Valley). This happened just over 5 million years ago; at that time, chunks of rocks of types that occur only up in the Rockies at the head of the Colorado River suddenly appeared in sediments of the Gulf of California, whereas before that time chunks of such rock types were absent in the Gulf of California.
You might think that with 5 million years of vigorous flow through the Canyon, the Colorado would have cut down even farther than it has, making an even deeper Canyon with a flatter, smoother river bed. But, the river has really had to cut the Canyon several times! Death-Valley-type Basin-and-Range faults associated with the opening of the Gulf of California also have cut across the Canyon, especially in the western end. Basaltic lava has come up some of the faults, in much the same way that the lava came up in Death Valley and in Red Canyon near Bryce. Several times over the last 5 million years, lava flows have dammed the Canyon, making lakes. Lakes accumulate sediment rather than eroding, while the erosive ability of the river is spent cutting through the dam. Once the river erodes the dam, it can then sweep out the loose sediment that accumulated in the lake, and then go back to deepening the Canyon… only to be dammed again by another lava flow. So, the river really had to cut down much more than a mile to make the canyon—cut down, get filled with lava, cut the lava, get filled again.
Why you don’t need to wait for a half-life to pass to measure it
In the text, you saw how radioactive decay occurs and provides “clocks” for the ages of rocks. Here, we go into a little more detail on the math, strictly for your entertainment and enjoyment.
The “law” of radioactive decay says that the more atoms of some radioactive parent you have, the more atoms of that parent will decay in some time. (There are many laws of this type: hotter bodies cool faster, rooms with more cats have more cats run out when you open the door, etc.). In addition, each radioactive parent type decays at its own particular rate, depending on the details of the quantum mechanics of its nuclear structure. Putting those words into math then goes like this. Given N parent atoms of some type, the change dN in the number of that type over some interval of elapsed time dt is:
The minus sign occurs because the number of parent atoms is decreasing over time as they decay to offspring. The K is a constant, called the decay constant (and often indicated with the Greek lambda, but we’ll stick with K). The numerical value of K is different for each different radioactive parent type, and includes the “physics” of how unstable the parent type is. A large K means a very unstable parent and a very rapid change to offspring; the units of K are inverse-time (so 1/seconds or 1/years).
If you never studied calculus, or you forgot what you studied, you won't make much sense of the next little bit. Don't worry. Those of you who took a calculus course and remember it will know that you can rearrange the equation to obtain:
Integrating yields:
in which ln indicates the natural logarithm, C is a constant that we will determine, and t is the total time that has elapsed. Taking the exponential of both sides, and noting that the decay started at some time t=0 when there were N=N0 parent atoms, yields the standard decay equation:
in which exp indicates the exponential (it usually appears as ex or exp or inv ln on calculators). The negative in front of Kt is equivalent to writing N=N0/exp(Kt). As t becomes large, exp(Kt) becomes very large, so N=N0/exp(Kt) becomes very small—the equation says that after a long time, you run out of parent atoms, which is correct.
Notice that if you can measure N0, wait for some time t1 and then measure N, the only unknown in this standard decay equation is K, so K can be calculated readily. The natural logarithm, ln, reverses the exponential so that ln(exp(-Kt))=-Kt. The natural logarithm appears on most calculators as ln or ln x or possibly as inv exp. Using this,
You usually will see this written as:
using one of the properties of logarithms.
We next estimate the half-life, t1/2. Note that after one half-life, N=N0/2. (So half of the parents have changed after one half-life.) If we let N=N0/2 in the standard decay equation, take the natural logarithm of both sides, remember that -ln(1/2)=ln2, and rearrange, we obtain t1/2=(ln2)/K. This is the basis for the statement in the text that you do not need to wait for a full half-life to pass if you wish to learn the half-life; you just need to start with N0, wait for any time t1, measure N, calculate K from this, and then calculate t1/2 from K. The half-life is useful, but most professionals in the field use the decay constant K most of the time, because K is more “fundamental” (it appears in the statement of decay given first above, and does not need to be derived as for the half-life).
Radiocarbon Revisited
You won’t have to look very far on the web to find sites—usually attached to certain religious ideas—complaining about errors in radiometric dating. (And Dr. Alley was once shown a published tract pointing out how stupid Dr. Alley himself must be to think that he could count more annual layers in an ice core than the total age of the Earth as estimated from writings in a particular religious text!) Some of the objections to radiometric dating are fairly silly, and even some of the young-Earth sites have put up notes asking followers to avoid using certain common arguments against scientists because those arguments are just wrong. The “5000-year-old” living clam falls in this category, as described later in this enrichment. The bottom line is that radiometric dating is useful, practical, successful, matches written records as far back as they go, matches other indications beyond that, and reveals a deep and fascinating history. Radiometric dating is not perfect, it does include errors, and practitioners have to know what they’re doing and think about it, but it works.
Skeptics about the use of scientific age dating in geology and the age of the Earth have especially focused on complaining about radiocarbon dating. This focus is odd, because radiocarbon—also called carbon-14—is not used in establishing the age of the Earth, or the age of the main geological events. The half-life of radiocarbon is only 5730 years; samples older than about 50,000 years have nearly run out of radiocarbon and so cannot be dated by radiocarbon. But, radiocarbon is used a lot in dating archaeological sites, and this may have caught the attention of people who study early written histories. In addition, as you will see, radiocarbon is more complex than many others (such as the potassium-argon system discussed in the regular text), and it may be easier to argue about complex things.
Much of the complexity of radiocarbon arises because the offspring of radiocarbon (the gas nitrogen-14) is very common, and is not retained well by the samples that are dated using radiocarbon (wood, charcoal, bone, or other formerly living things—not most rocks). Thus, radiocarbon dating does not look at the parent-to-offspring ratio; instead, the starting concentration of radiocarbon is estimated, the concentration today is measured, and the ratio gives the age. Radiocarbon is mostly made in the atmosphere, when cosmic rays collide with atoms and knock off neutrons that then hit nitrogen-14 nuclei and make carbon-14. This doesn’t happen very rapidly; natural production is just about 15 pounds for the whole Earth per year, or just over two carbon-14 atoms per square centimeter (just under 1/2 inch on a side) of the Earth’s surface per second.
In the atmosphere, radiocarbon quickly combines with oxygen to make carbon dioxide. The atmosphere is well-mixed—release some gas molecules here, and within a few years they will be spread fairly uniformly around the planet—so the radiocarbon-bearing carbon dioxide is quite uniformly distributed around the globe. Green plants grow by using carbon dioxide, and roughly one of each trillion carbon atoms in the atmosphere and in green plants is carbon-14 rather than stable types of carbon-12 or carbon-13. Plants are eaten by animals. Most animals live less than 100 years, whereas most carbon-14 lasts thousands of years, so when plants and animals die, they have just about the same ratio of carbon-14 to carbon-12 as was in the atmosphere when they were still alive. After plants or animals die, they do not breathe or eat any more, so they don’t take in carbon-14 while the carbon-14 in them decays. Hence, the ratio of carbon-14 to carbon-12 in a formerly living material is a clock.
Whole textbooks can be written refining the previous two paragraphs, and a scientific journal, Radiocarbon, focuses almost exclusively on the topic. If you aren’t a real stickler for accuracy—if “this died sometime between 9,000 and 11,000 years ago” is good enough for you—then you really don’t need a whole journal devoted to radiocarbon. (You still need to worry about one or two things that we’ll come to, but not about too many.) But if you want to get the answer right to within a few decades or less, then you have to be really careful.
One problem is that production rates of radiocarbon have varied over time. When the sun is more active or the Earth’s magnetic field is stronger, they protect us more from cosmic rays and reduce production of radiocarbon. The changes are not huge, and there are ways to correct for them (changes in the strength of magnetization can be estimated by measuring the degree of alignment of the “magnets” in lava flows or sediments of different ages, and the activity of the sun can be tracked from the magnetic measurements plus the ice-core concentrations of beryllium-10, which is also made by cosmic rays).
Changes in the Earth’s carbon cycle also matter a little to the history of the starting concentration of carbon-14 in plants and animals in the past. For example, now we are pulling up immense quantities of really old fossil fuels that do not have any remaining carbon-14, and burning those fossil fuels to make carbon-14-free carbon dioxide that goes into the atmosphere, diluting the carbon-14 there. When we humans were busily blowing up atomic bombs in the atmosphere, they made a lot of carbon-14. Before we were so influential, changes in carbon-14 in the atmosphere were MUCH smaller, and changes in ocean circulation were probably most important—some carbon dioxide goes from atmosphere to ocean, and the ocean waters sink in certain places and spend a thousand years or so down deep before coming back up to exchange carbon dioxide with the atmosphere. Because some of the carbon-14 from the atmosphere ends up decaying in the deep ocean, the ocean circulation actually reduces atmospheric radiocarbon—if water didn’t sink into the deep ocean, there would be less carbon-14 there and less carbon-14 decay there, and that would leave more carbon-14 in the atmosphere. At certain times in the past, less sinking of ocean waters seems to have occurred, allowing more carbon-14 to exist in the air.
The usual way to handle all of this is to use radiocarbon to date tree rings (which quit exchanging carbon with the atmosphere as soon as they grow) or shells in annually layered sediments, and use the layer-counted ages and the known half-life of radiocarbon to calculate the starting concentration of radiocarbon. Because radiocarbon is well-mixed in the atmosphere, and must have been well-mixed in the past, a calibration curve developed from samples anywhere on Earth can be used for samples from anywhere else. You can also date some samples, such as corals or cave formations, using two techniques: an accurate technique such as uranium-series disequilibrium, and radiocarbon, and so obtain a calibration curve for the radiocarbon. Many different calibration studies have been conducted, and while they do not agree perfectly and research is ongoing, they agree reassuringly well. The biggest corrections are a bit more than 10% with uncertainties of less than 1%—a sample that looks to be 10,000 years old, assuming that there were no changes in radiocarbon concentration of the atmosphere, is actually about 11,500 years old, because the radiocarbon concentration of the atmosphere did change, and the uncertainty in this is less than 100 years.
If you are primarily interested in the question “Does the world really look older than written records”, even radiocarbon provides a very good answer (“Yes, with very high scientific confidence”). Science has long since moved past that question, and the research frontier involves numerous fascinating questions, such as whether we can reconstruct changes in ocean circulation from the changing calibration of the radiocarbon clock after correcting for the changes in the sun and the magnetic field.
Plants actually have a slight preference for carbon-12 over carbon-13 or carbon-14 (the lighter atoms diffuse into the plant and react more easily), so the concentration of carbon-14 in a plant is slightly less than the concentration in the air. The preference for carbon-12 over carbon-13 is half as big as the preference for carbon-12 over carbon-14, so measuring the concentrations of all three types allows an accurate correction; this measurement is made easily and is done routinely when highly accurate dates are needed, so it should not bother anyone much.
The 5000-year-old living clam raises a different but interesting issue. All of the discussion so far has assumed that the items being dated obtained their carbon from the atmosphere. That is almost always a pretty good approximation for almost everything. But suppose that you “ate” only things that had been dead for a long time—you would not take in much radiocarbon, and so you would look old to someone who assumed that you ate things containing normal concentrations of radiocarbon. Certain special ecosystems on the sea floor do just that; they live on natural oil seeps, eat the oil or eat things that ate the oil, and the oil is old and so lacks radiocarbon. If you were stupid enough to sample these and assume that they were eating “normal” foods, then you would mistakenly assume that the living creatures had been dead for a long time.
Such oil-seep ecosystems are quite rare and special. A more-common situation is a clam in a creek in a carbonate terrain. When caves are being made, the chemical equation for the water and carbon dioxide dissolving the rock is:
H2O+CO2+CaCO3→Ca+2 +2HCO3-
The rain and atmospheric carbon dioxide on the left of the equation combine with the calcium carbonate of the limestone, yielding the calcium and bicarbonate ions on the right-hand side of the equation that are freed to wash down the creek. If a clam is making its CaCO3 shell from the water, the clam just runs this reaction backward. Notice, however, that half of the carbon, C, in the water came from the atmospheric CO2 and half from the rock. The rock is almost always very old, and has no radiocarbon. So, a clam in this situation would form a shell with only half as much radiocarbon as for a clam growing in a stream that does not drain carbonate rocks and that gets all of its carbon from the atmosphere. Hence, if scientists were clever with their instruments but stupid otherwise, those scientists might end up thinking that a living clam had been dead for over 5000 years.
Scientists are fully aware of this. For decades, however, there was a convention of reporting all radiocarbon measurements as the equivalent age assuming that the sample had been in equilibrium with the atmosphere. Dr. Alley is reasonably confident that the myth of the clam that was living yet the scientists thought it was thousands of years old came from work by a distinguished senior colleague, who in the 1960's published papers listing dates in the conventional fashion. That colleague actually was using the results to learn about the geochemistry of the waters. As noted above, some of the young-Earth-creationist websites have asked their supporters to “clam up” about this, because using it in an attempt to discredit scientists instead makes the young-Earth-creationists look confused.
As a possibly interesting aside, the natural flavoring vanilla is obtained from the pods of a tropical orchid, but the main chemical in vanilla can also be obtained from petroleum much more cheaply. This creates an incentive for cheaters to sell petroleum-extracted vanilla as the real thing. Cheaters can be caught, though, because real vanilla contains radiocarbon but the fossil-fuel version does not. When Dr. Alley was writing this, commercial testing for a small fee was available to protect consumers and natural-vanilla producers.
Optional Enrichment: Widening and Narrowing
Video: Widening and Narrowing (2min 22sec)
Back in Module 5, you learned about landslides and rockfalls. In Module 6, you saw that rivers can cut down, but this makes steep slopes that can experience those landslides and rockfalls. This produces V-shaped river valleys. With all this knowledge, you could have played professor and explained the shape of the Grand Canyon to a tourist who had not worked through those earlier Modules. Here’s how we explained it with the CAUSE class. See how you do…
Click here for a transcript of the Widening and Narrowing video.
I was out at the Grand Canyon a few years ago. And I was standing behind the fence there. And I was talking to a very nice and knowledgeable ranger about the history of mining at the place. And we were looking at those incomparable cliffs. And there's sort of a cliff of limestone, and a slope of shale, and a cliff of sandstone on another slope, and so on, on down to the river sitting way down below someplace like that.
And while we were standing there talking, this gentleman walked up, and he asked us why the river had gotten narrower. And we gave him a confused look. And he said, well, the river down there is very narrow. But if you try to look all the way across to the North Rim which is way the heck over there, what you see is, in fact, that the top is very broad. So he sort of figured that the river had been wide, and then it had gotten narrow.
Now, right beyond the fence there was a bit of a crack down into the cliff-forming rock there. And I ask him whether he would have any interest in going out beyond that crack and taking a jackhammer and starting to work on it. And he offered the opinion that eventually it would break off. And that when it broke off, that he would end up somewhere down the slope with a big rock on top of him, and that that would not be a good thing to do.
Well, then I ask him if he looked across the canyon, did he see places where a lot of rocks had piled up that looked like they had fallen off of the cliff. And he said, well, yeah I do. And that one fell. And this was a bright person. He immediately got it. He says, oh, what happens then is that the river must cut down. And once the river has cut down some, then, this slow process of mass wasting is going to widen it.
And in particular what happens is that the shales, the slope-formers cut down. And that makes the cliffs higher. And if the cliffs get really high, they tend to fail, and blocks fall off. And so that after a while you look at it, and you find that the canyon sort of has the same shape that it used to. But it's gotten wider as well as getting deeper as the river comes down.
Module 10 Wrap-Up
Module 10 Wrap-Up
In Module 10, you have taken a virtual hike to the bottom of the Grand Canyon, and made it safely back up, doing geology all the way. You have learned to estimate when events occurred in the past by using annual layer counts, “uniformitarian calculations” from the nature of the rocks, the time needed to form such rocks and radioactive dating. You know a little more about the very long, fascinating history of the Earth.
For many of you, this 4.6 billion-year history is pretty obvious now. You learned it first in elementary school, had that reinforced a few times since, and now we’re just repeating things you don’t need to have repeated. But for some of you, this is a major issue, because you have never learned it, or you were told not to learn it, or you otherwise have real issues with it. We have provided a lot of information in the main Module, and a lot more in the Enrichment, to try to give all of you a solid background so that everyone can do well on the RockOn Quiz.
Please note that if you still reject the geology, that’s fine, we can’t force you, and we don’t want to force you. But, you should give the geologically accepted answers on the RockOn Quiz, to show that you have understood the material. Those answers are that the Earth has a long, complicated history, and that there is no serious scientific argument about this.
Review Module Requirements
You have reached the end of Module 10! Double-check the list of requirements on the Welcome to Module 10 page and the Course Calendar to be sure you have completed all the activities required for this module.
Reminder
Continue to work on Exercise #5. See the Course Calendar for specific dates.
Comments or Questions?
If you have any questions, send an email via Canvas, to ALL the Teachers and TAs. To do this, add each teacher individually in the “To” line of your email. By adding all the teachers, the TAs will be included. Failure to email ALL the teachers may result in a delayed or missed response. For detailed directions on how to do this, see How to send an email in GEOSC 10 in the Important Information module.
