Click here for a transcript of the Properties of Matter: Alloys and Their Properties video.
In this video we see how different metals bond together to form alloys which still retain the metallic properties of the starting metals but are usually stronger. Metal atoms are typified by having only a few electrons in their outer shells. This means that even when they bond there's always room in this valence shell for more electrons. Each metal atom can bond with up to 12 others in the close-packed lattice. Look at the red atom. It is surrounded by six in its plane and three on top and three underneath.
A less compact crystal structures are possible too. For example, this arrangement where each atom is bonded to eight others. Because there are still not enough electrons to complete the outer shell any of the atoms the electrons can move easily from one atom to another making metals good conductors of both electricity and heat. And because the electrons are not localized in fixed bonds, the atoms can slide past each other making them ductile allowing the metal to change shape. It also means that when you try to react metals together the atoms normally just mix into the lattice forming metallic bonds with each other and with no fixed proportions and randomly distributed. These structures are called alloys. Contrast this with compounds between metals and nonmetals or between nonmetallic elements where the proportions of each element is fixed.
The oldest example of an alloy perhaps is the way bronze took over from copper in the early human communities of Europe around 6,000 years ago. During the late Stone Age, axes began to be made of pure copper but they were fairly soft. When small amounts of tin were added to make bronze you got an ax which was twice as hard and worked well. The Bronze Age had arrived. The atoms in a metal lattice are held by non-directional bonds a sort of sea of loose electrons as we said allowing the atoms to slide past each other still touching making metals relatively easy to melt and bend but hard to vaporize. When metals change shape atoms actually slip over each other like this. However, this process does not happen all at once but bit by bit rather like trying to move a carpet by putting a rock in it.
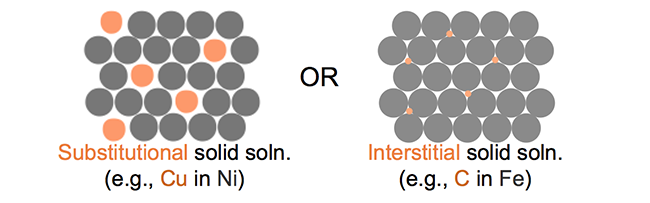
Here is the way it happens in the metal. You see the slip moving easily one atom at a time where there's a dislocation in the lattice. It is this easy movement of atoms in the crystal lattice that makes most pure metal soft. Now put a smaller or bigger atom into the lattice and this easy movement of the dislocation is blocked. See the way the bigger atom stabilizes the dislocation which gets no further unless you put greater force meaning that it's harder to bend the alloy.
To finish let's look at some well-known alloys. Bronze, three quaters copper, quarter tin, for sculptures, boat hardware, screws, and grille work. Brass 70 percent copper, 30 percent zinc. Musical instruments, coins, door knockers. Carbon steel 99 percent iron and up to one percent carbon. The building construction, tools, car bodies, machinery rails, etc. stainless steel iron with about 18 percent chromium and eight percent nickel. Used for tableware, cookware, surgical tools, and so on. Aluminium alloys for planes contain a few percent of copper or other metals. Amalgam is mercury with silver and other metals. Once used for dental fillings. Solder lead and tin for joining electrical wires and components. Melts very easily. Gold is usually an alloy containing another metal such as silver for increasing hardness. The number of carats, k, defines how many mass parts of pure gold are found in 24 parts of the alloy.